





From Paper to Pill
What often starts as a sketch on a piece of paper must undergo a rigorous cycle of trial and error until, ultimately, a new therapy is born. That’s the iterative nature of small molecule drug discovery – and it begins with identifying which target to go after.
Understanding new ways to go after established targets is essential to improve how we treat cancer. One such target is hypoxia-inducible factor (HIF)-2α.
HIF-2α is a type of protein called a transcription factor. Transcription factors help control which genes are turned on or off at the appropriate times. Normally, HIF-2α helps cells respond to low oxygen levels in their environment.1 However, it also plays a role in cancer. For many years, HIF-2α has been known to be highly active in certain cancers when it shouldn’t be.2 This protein induces biological processes that promote cancer growth, such as blood vessel development and cell proliferation.3 But, before HIF-2α can act on its gene targets and cause cancer cell growth, it must bind to its partner – a protein called HIF-1β.3
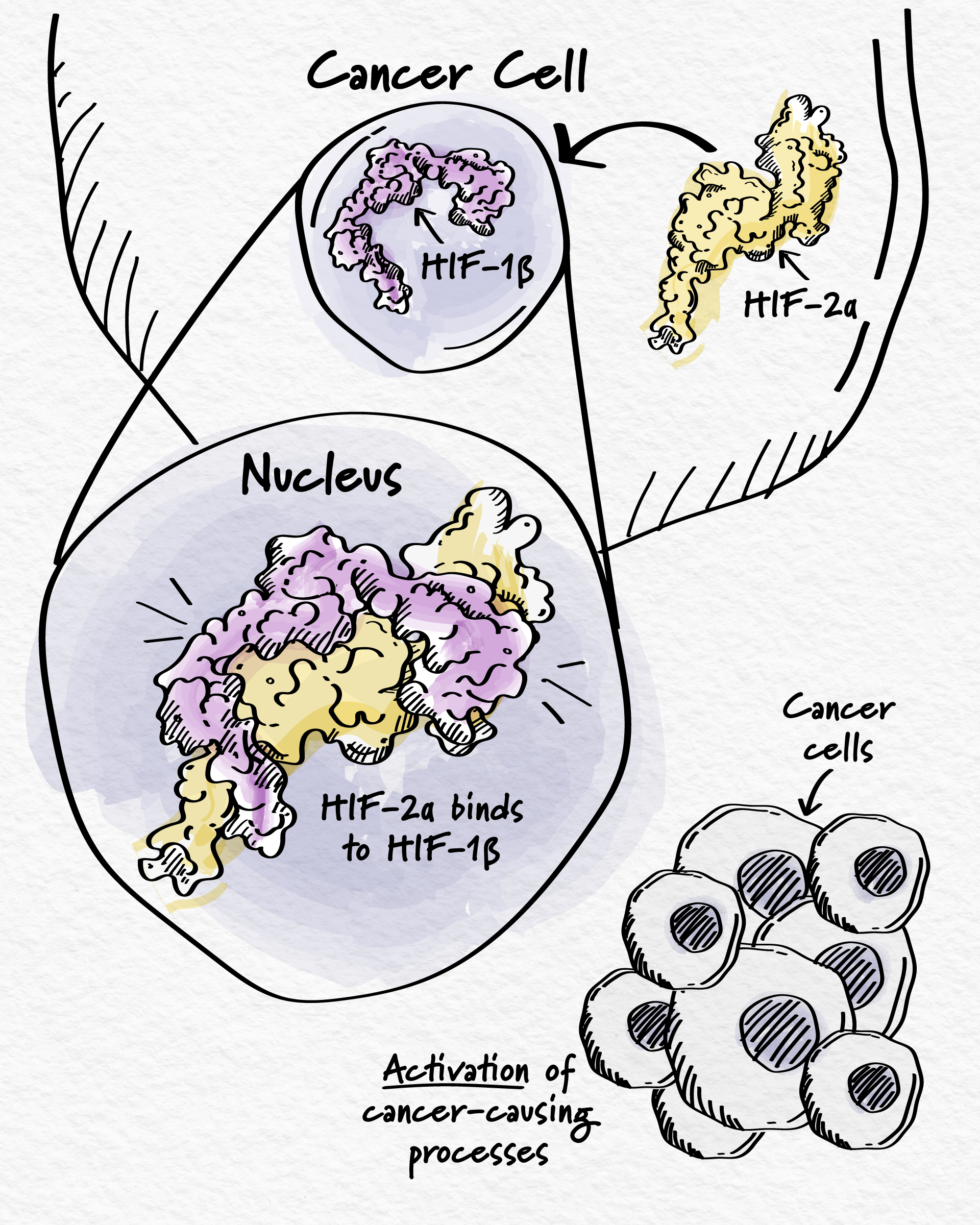
Shapeshifter
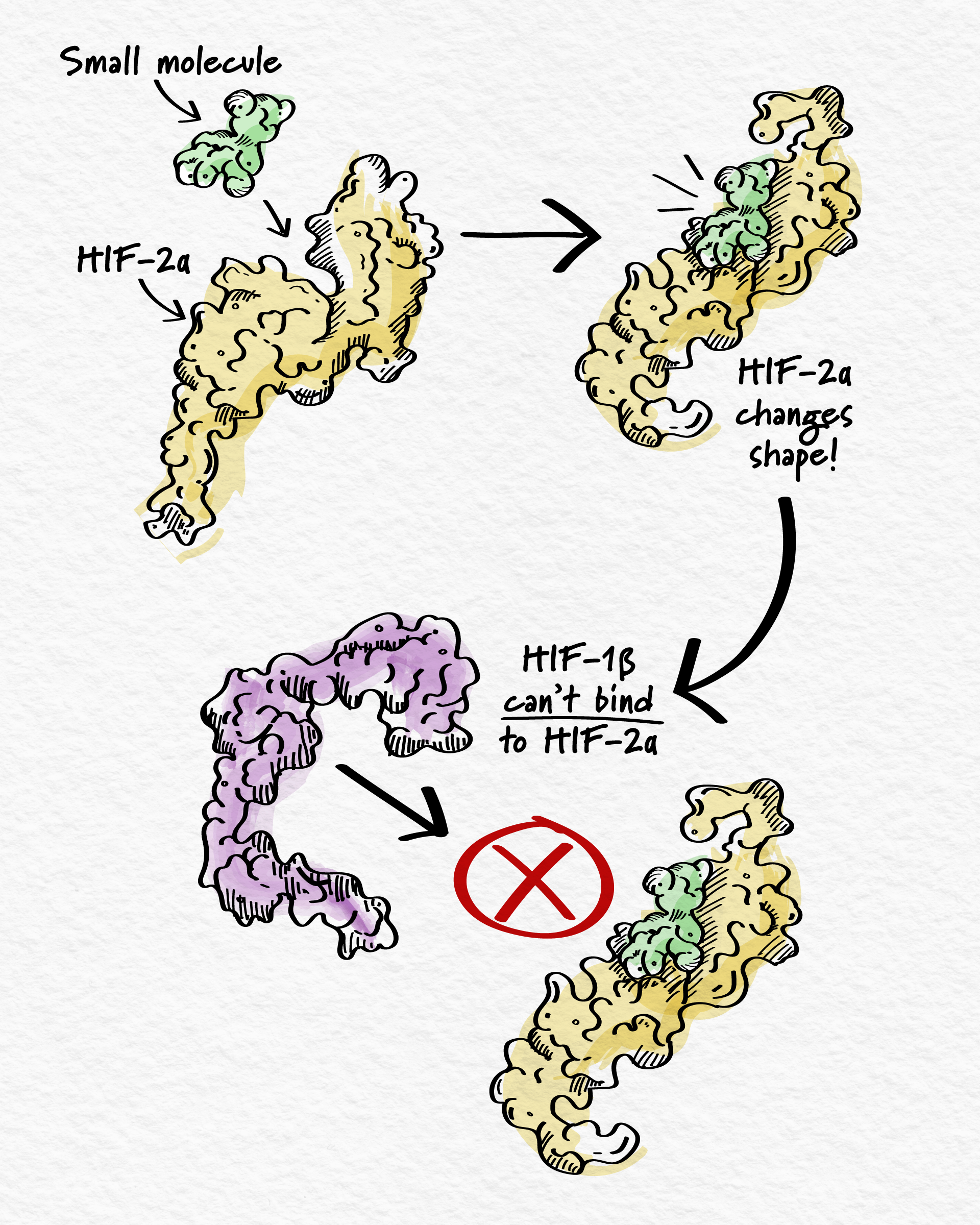
To stop HIF-2α from promoting cancer growth, the goal is to prevent it from binding to HIF-1β. But that involves blocking the interaction between two proteins, which is usually not an easy task due to the large surface across which they interact. However, HIF-2α is unique – it has a distinctive pocket suitable for a small molecule to bind.4 Once bound, this causes HIF-2α to change shape and no longer bind to its partner HIF-1β – inhibiting gene activation and, in turn, cancer cell growth.4,5,6
This approach to blocking HIF-2α has been validated and one drug was approved to treat certain cancers after demonstrating incremental improvements in patient outcomes.7 However, there is opportunity to develop new and potentially improved molecules that block HIF-2α to further improve outcomes.
Diligent Design
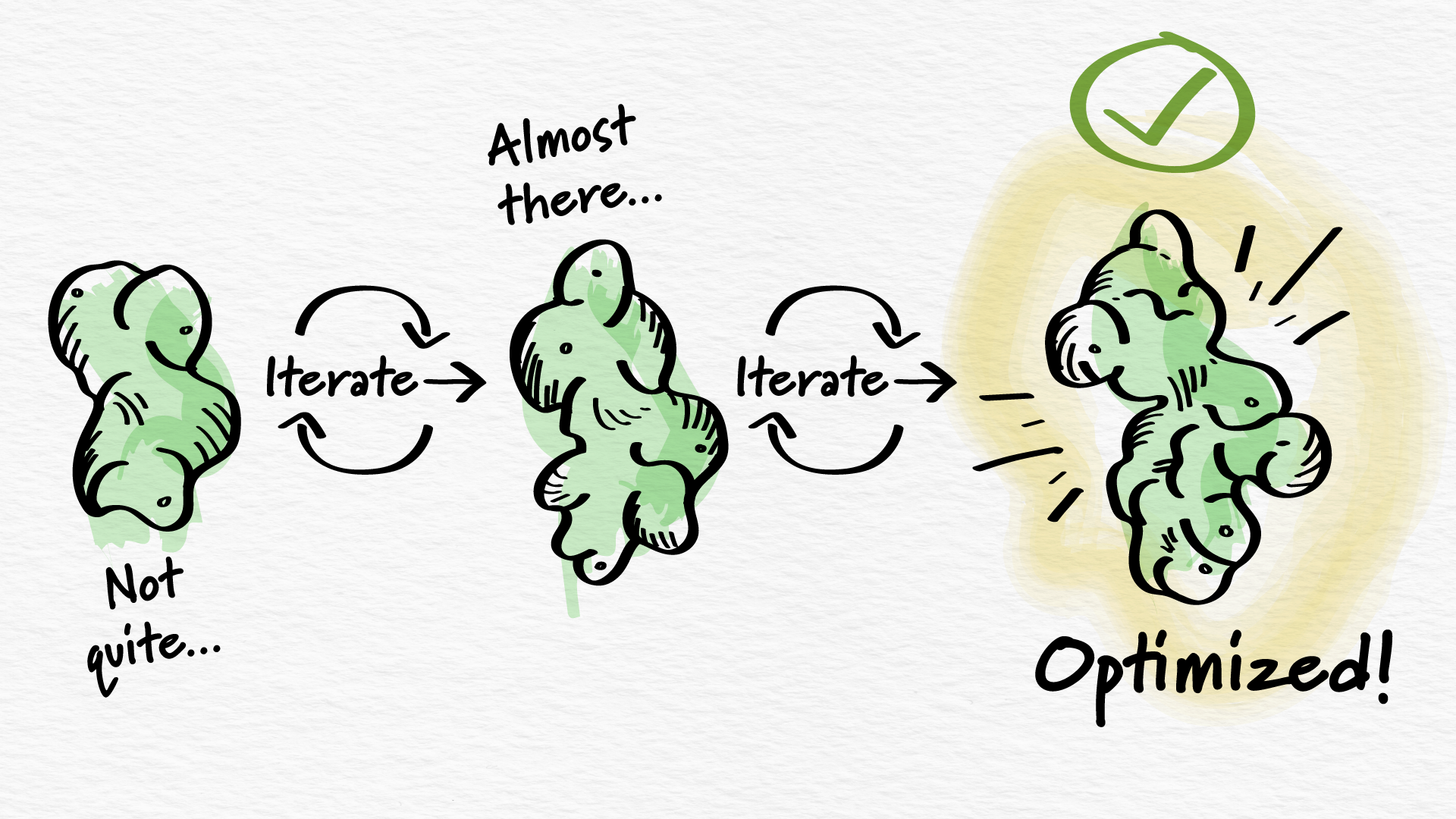
Building from scratch a new molecule that could change the way patients are treated requires immense attention to detail. Arcus’s small molecule team meticulously and deliberately added specific atoms to gradually grow and develop the ideal molecular candidate, initially identified as AB521 and now known as casdatifan.
The process of making casdatifan required a far greater number of synthetic steps than is often required to build a small molecule, but this investment was essential to Arcus’s goal of developing best-in-class treatments against well-characterized biological targets. In its final form, casdatifan was carefully constructed to stably and specifically fit in the sphere-like pocket of the HIF-2α protein.
Once casdatifan was engineered, researchers turned to understanding what the body does with it, as well as what casdatifan does to the body. This vital information helps scientists deliver the small molecule at an optimal dose to potentially improve clinical outcomes while maintaining a favorable side effect profile, an important characteristic for combining casdatifan with other novel medicines in the future.
A key goal in the casdatifan development process was to optimize several properties to address unmet needs in the current treatment landscape. This included exposure, which is the amount of the molecule that gets absorbed into the bloodstream and distributed to various tissues. By optimizing these properties, the Arcus team hoped to target HIF-2α more completely, including in hard-to-reach tissues, like cancer lesions.
Up to the Test
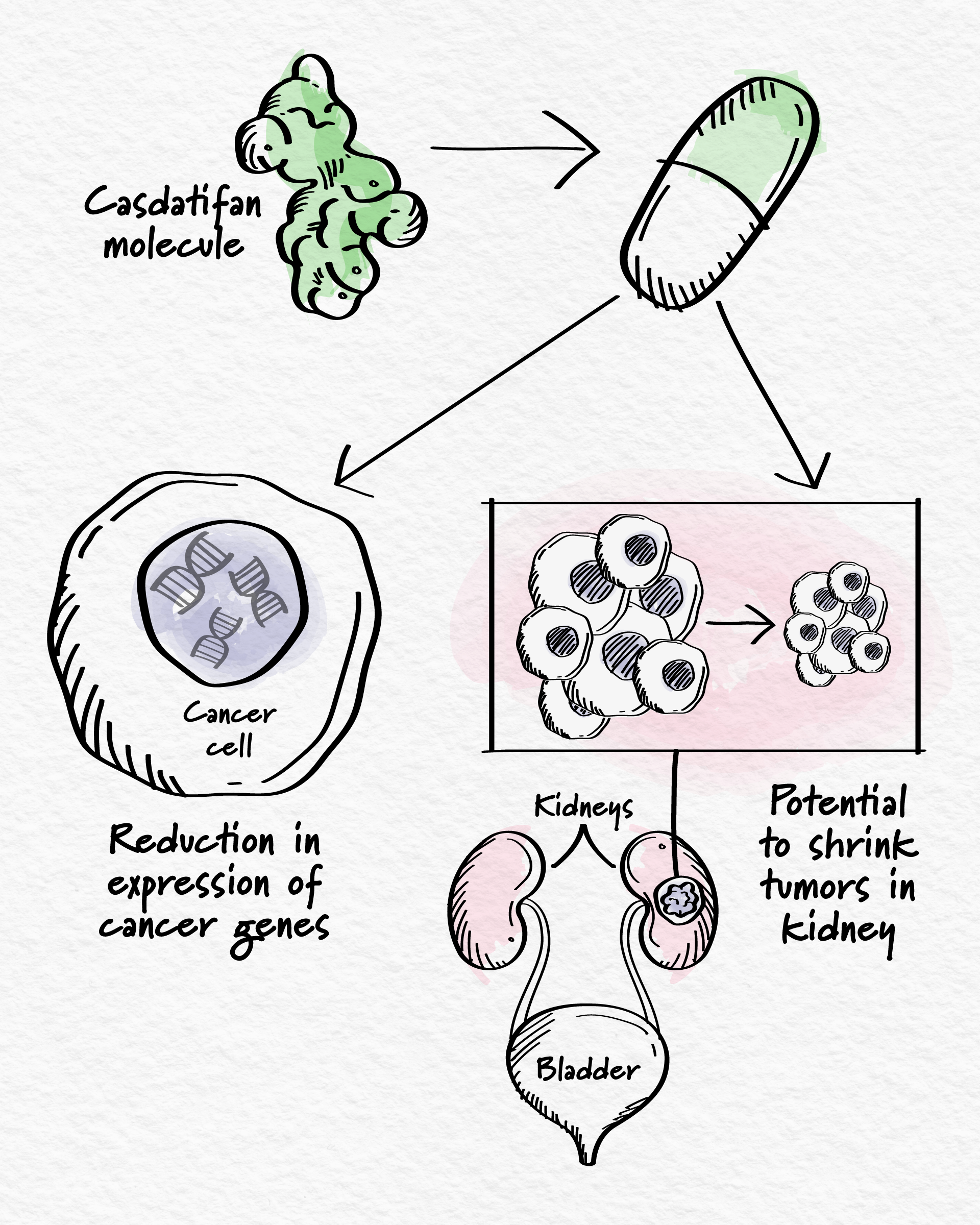
Once the small molecule team was satisfied with the design and characterization of casdatifan through a meticulous, iterative process, it moved on to the next phase of development, in which scientists could observe the molecule at work in a preclinical setting. The team was encouraged to see how strongly it bound to the HIF-2α pocket, reduced the expression of genes that promote cancer growth, and led to antitumor activity in animal models.8,9 These results indicated that the molecule was appropriately designed to impact the appropriate biological pathways.
Now, casdatifan has advanced to the ultimate challenge of drug development – clinical trials. As with all of our molecules, casdatifan is being investigated to address specific clinical settings where the disease biology aligns with the specific therapeutic hypothesis for the molecule. In this case, that was clear cell renal cell carcinoma (ccRCC), a type of kidney cancer in which a gene that normally controls HIF-2α is mutated, leading to increased HIF-2α activity.2
At the ASCO Annual Meeting, new Phase 1/1b clinical trial results show that casdatifan has promising antitumor activity, demonstrating its potential to shrink tumors and slow disease progression.
These results are the first signs that casdatifan has clinical potential for what Arcus scientists designed it to do.
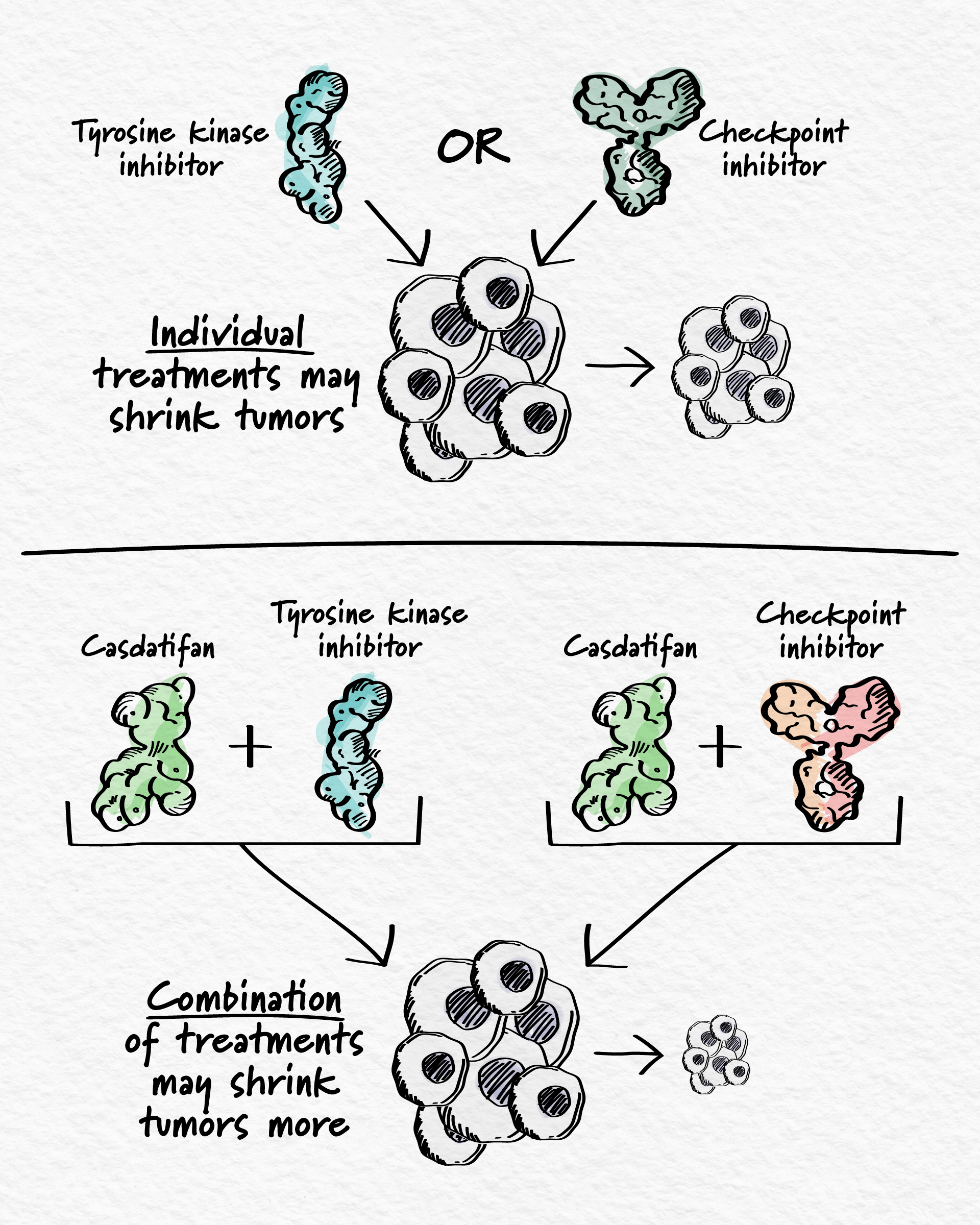
Combining Casdatifan
Casdatifan was thoughtfully designed to maintain a favorable side effect profile, making it a promising combination partner for new treatment options in oncology. With encouraging clinical data supporting its potential future use in people, combination approaches using casdatifan are underway to address the complex underlying biology of ccRCC.
Our combination strategies are based on treatments that are known to work individually in kidney cancer but where there is still room to improve clinical outcomes. Today, casdatifan is being investigated in combination with a tyrosine kinase inhibitor (ARC-20, NCT05536141), which also blocks several cancer-driving processes and, separately, with an anti-PD-1/CTLA-4 immunotherapy (eVOLVE-RCC02, NCT07000149) that simultaneously blocks two checkpoint inhibitors, allowing the body’s own immune system to find and attack cancer cells. In the first half of 2025, we plan to initiate a Phase 3 study (PEAK-1, NCT07011719) of casdatifan with a tyrosine kinase inhibitor.
Arcus’s casdatifan combination trials currently focus on patients who are just starting their first treatment post-diagnosis, or those who have previously used immunotherapy. By combining casdatifan with different combination partners and in different lines of treatment, the goal is to stop multiple cancer-driving processes simultaneously across the spectrum of ccRCC patients with unmet need.
What began as a sketch on a piece of paper has gradually risen to a promising new approach for cancer patients in need through the insights of our small molecule discovery team and their close collaboration with other research teams. With encouraging data so far, casdatifan may have the potential to change the way HIF-2α is inhibited and is a strong candidate for investigational combination regimens that could change the way kidney cancer is treated.
Casdatifan is an investigational molecule. Arcus has not received approval from any regulatory authority for any use globally, and its safety and efficacy for the treatment of renal cell carcinoma has not been established.
References:
1. Jaśkiewicz, Maciej, et al. “The transition from HIF-1 to HIF-2 during prolonged hypoxia results from reactivation of PHDs and HIF1A mRNA instability.” Cellular & molecular biology letters 27.1 (2022): 109.
2. Cuvillier, Olivier. “The therapeutic potential of HIF-2 antagonism in renal cell carcinoma.” Translational andrology and urology 6.1 (2017): 131.
3. Davis, Leah, et al. “Targeting HIF-2α in the tumor microenvironment: redefining the role of HIF-2α for solid cancer therapy.” Cancers 14.5 (2022): 1259.
4. Scheuermann, Thomas H., et al. “Artificial ligand binding within the HIF2α PAS-B domain of the HIF2 transcription factor.” Proceedings of the National Academy of Sciences 106.2 (2009): 450-455.
5. Wallace, Eli M., et al. “A Small-Molecule antagonist of HIF2Α is efficacious in preclinical models of renal cell carcinoma.” Cancer Research, 76.18 (2016), 5491–5500.
6. Cho, Hyejin, et al. “On-target efficacy of a HIF-2α antagonist in preclinical kidney cancer models.” Nature 539.7627 (2016): 107-111.
7. WELIREG (belzutifan) [prescribing information]. Rahway, NJ: Merck Sharp & Dohme LLC; 2021-2023.
8. Foster, Paul, et al. Targeting Hypoxia Inducible Factor (HIF)-2α With AB521, a Novel and Potent Small Molecule HIF-2α Inhibitor, for the Treatment of Clear Cell Renal Cell Carcinoma. Poster presented at: AACR Special Conference: Advances in Kidney Cancer Research; June 24-27th, 2023; Austin, Texas, USA.
9. Lawson, Kenneth, et al. Discovery and characterization of AB521, a novel, potent, and selective hypoxia-inducible factor (HIF)-2α inhibitor. Poster presented at AACR Virtual Annual Meeting 2021; April 10-15th, 2021; Online.
RECENT ARTICLES
Combining for Change in Kidney Cancer
While there has been substantial progress in treatment in recent years, even more innovative approaches are needed to improve survival, especially when the cancer is metastatic, meaning it has spread to other parts of the body.
Addressing Adenosine: Immunotherapy in the Tumor Microenvironment
Targeting the adenosine axis – components that contribute to the production of adenosine and its subsequent impact – is a promising option.

