Investigating Combination Therapies to Treat Cancer
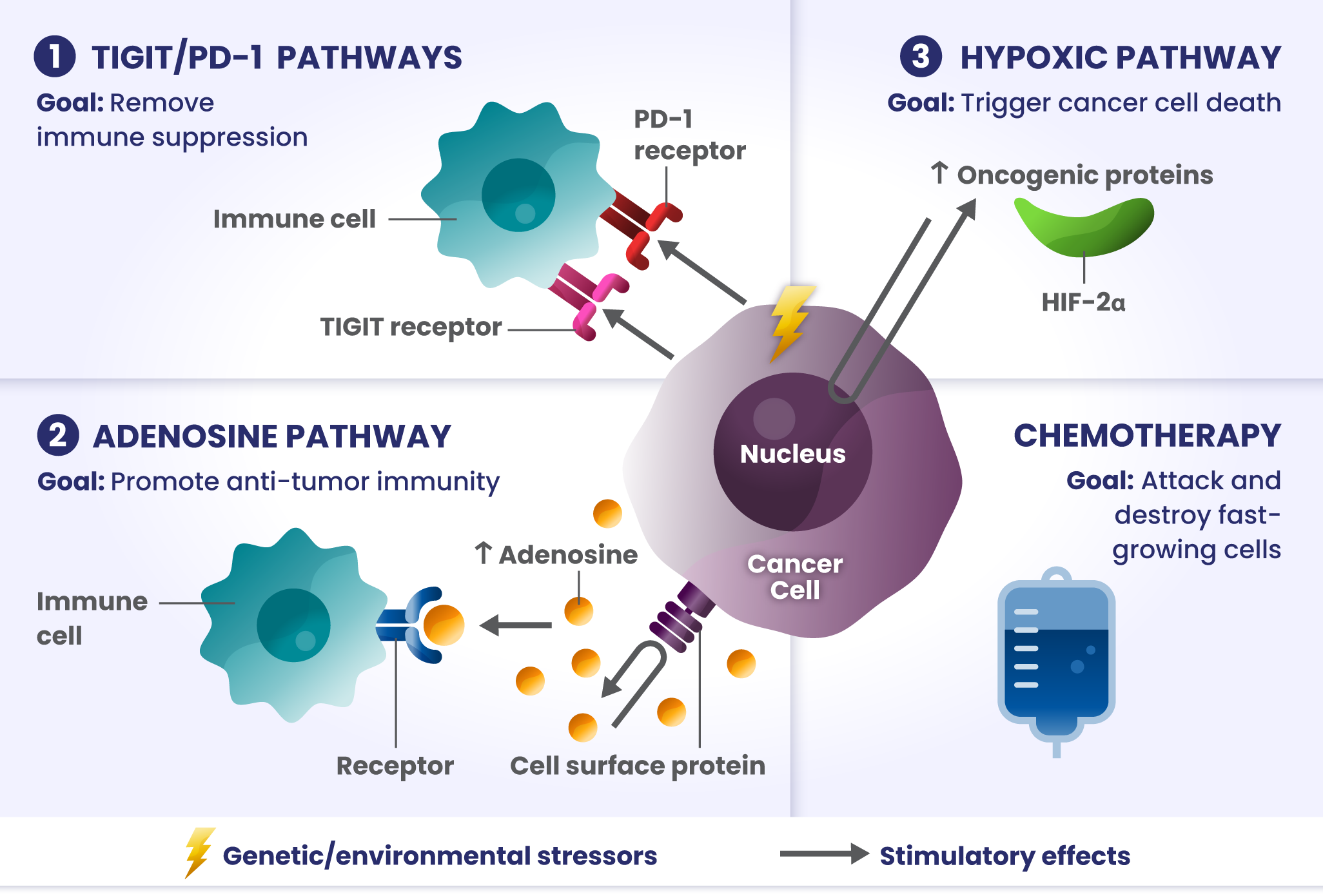
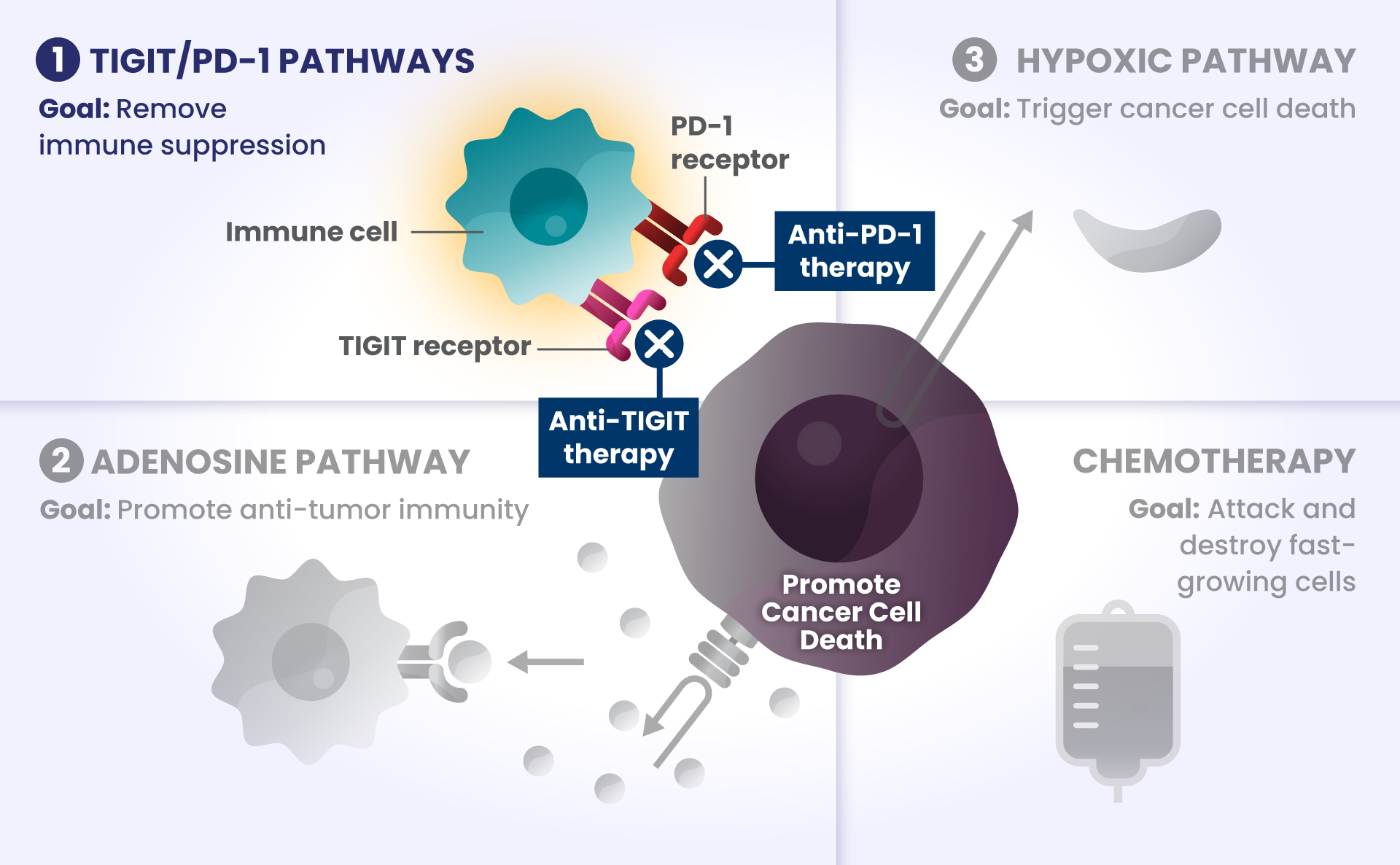
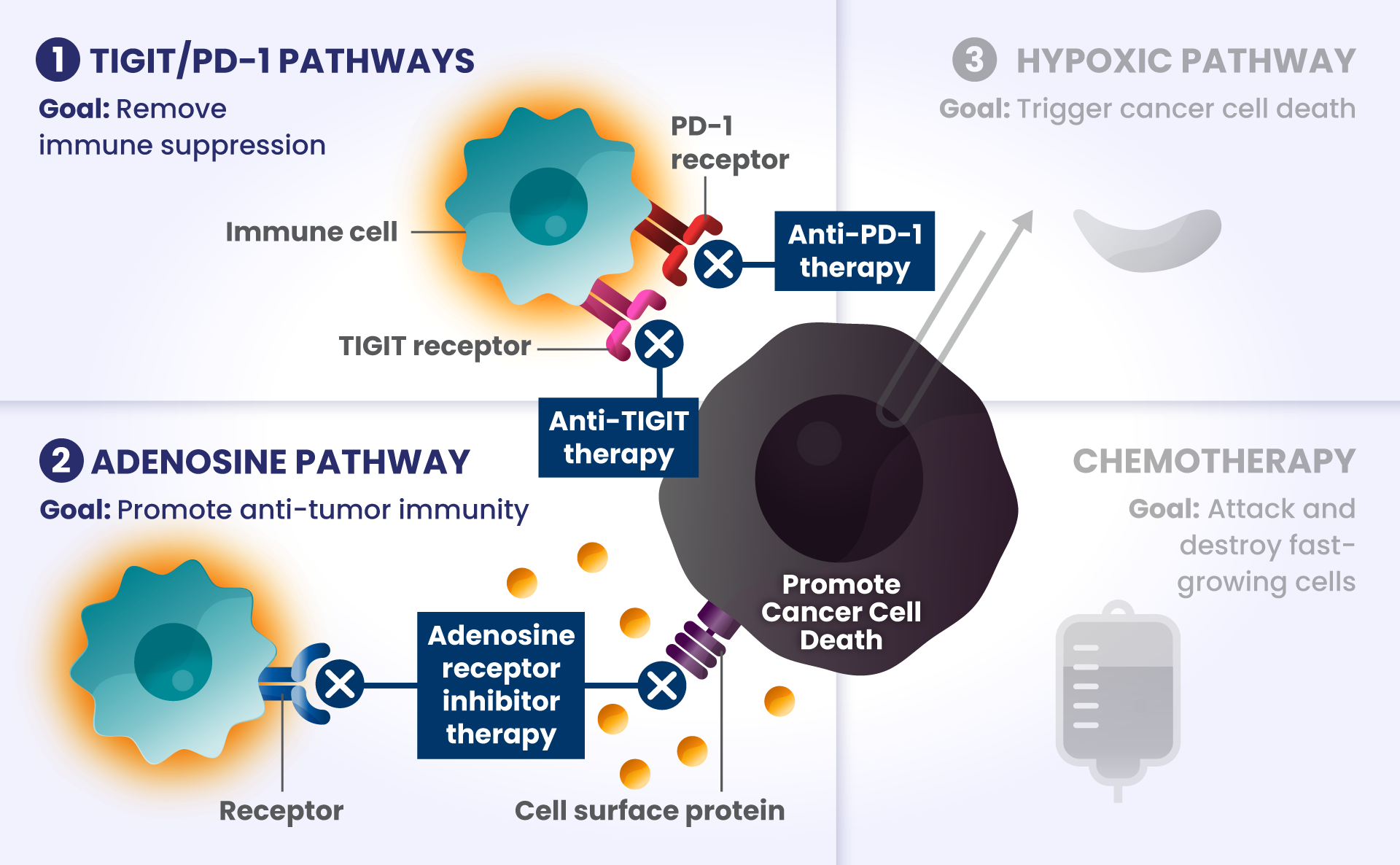
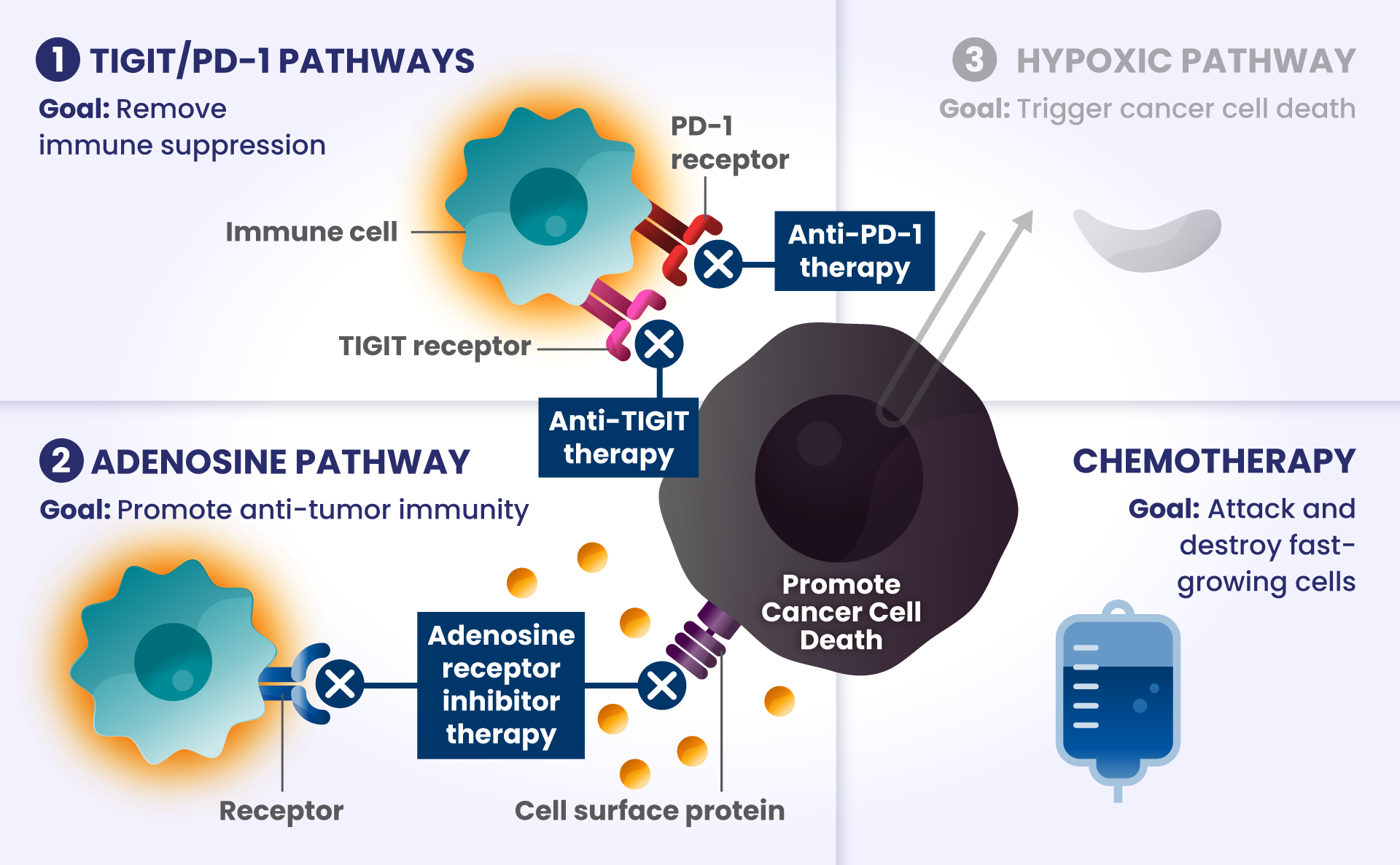
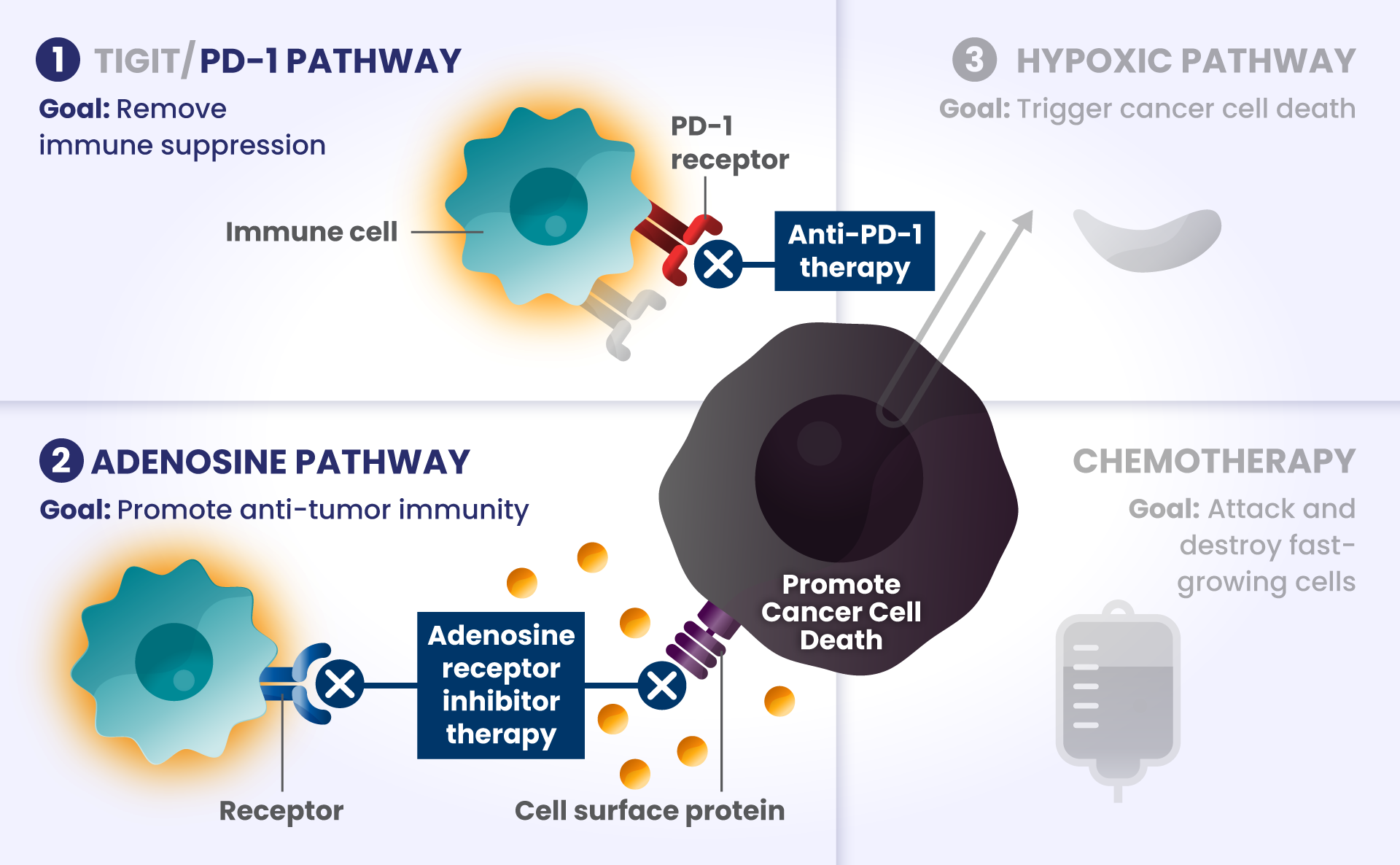
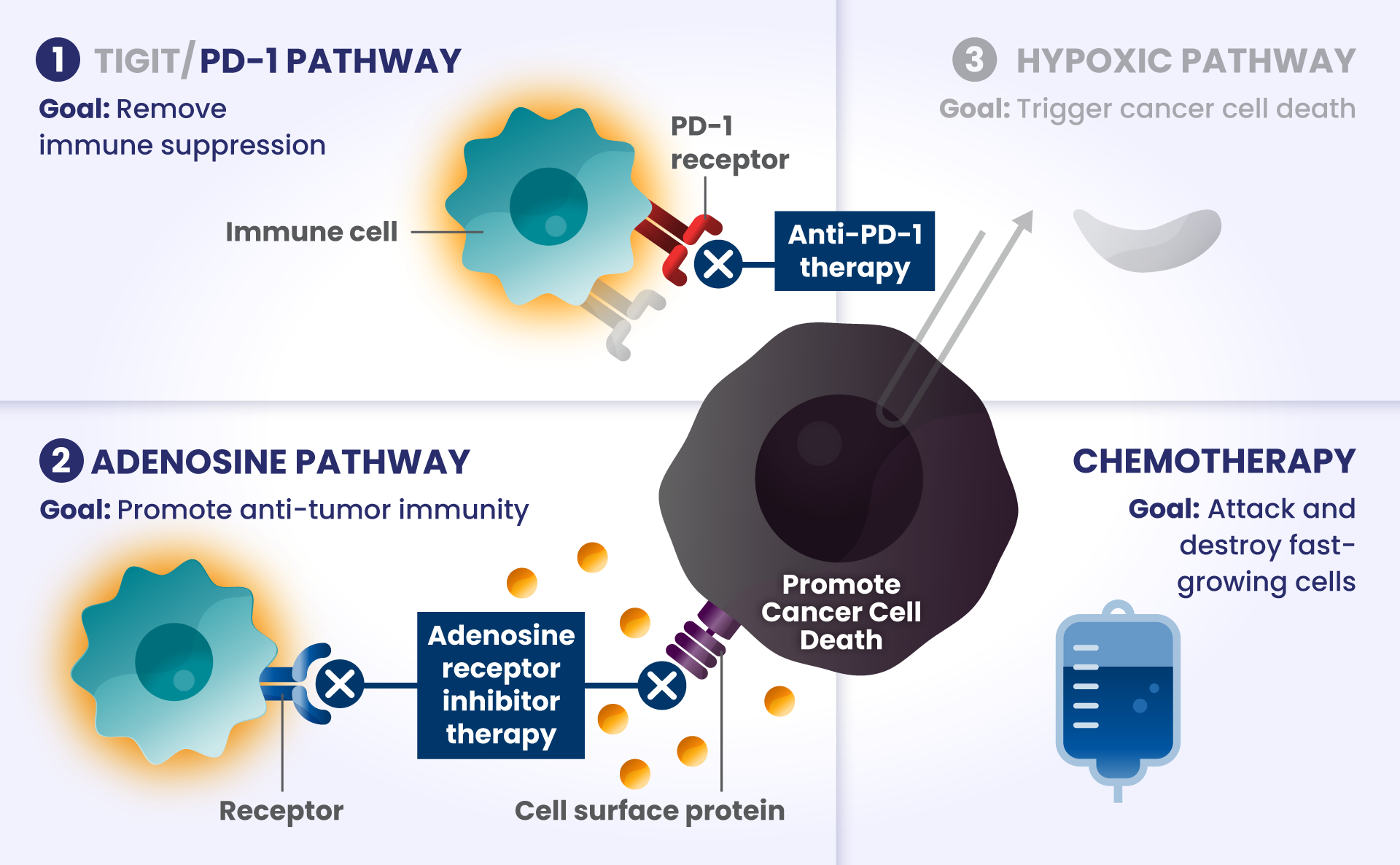
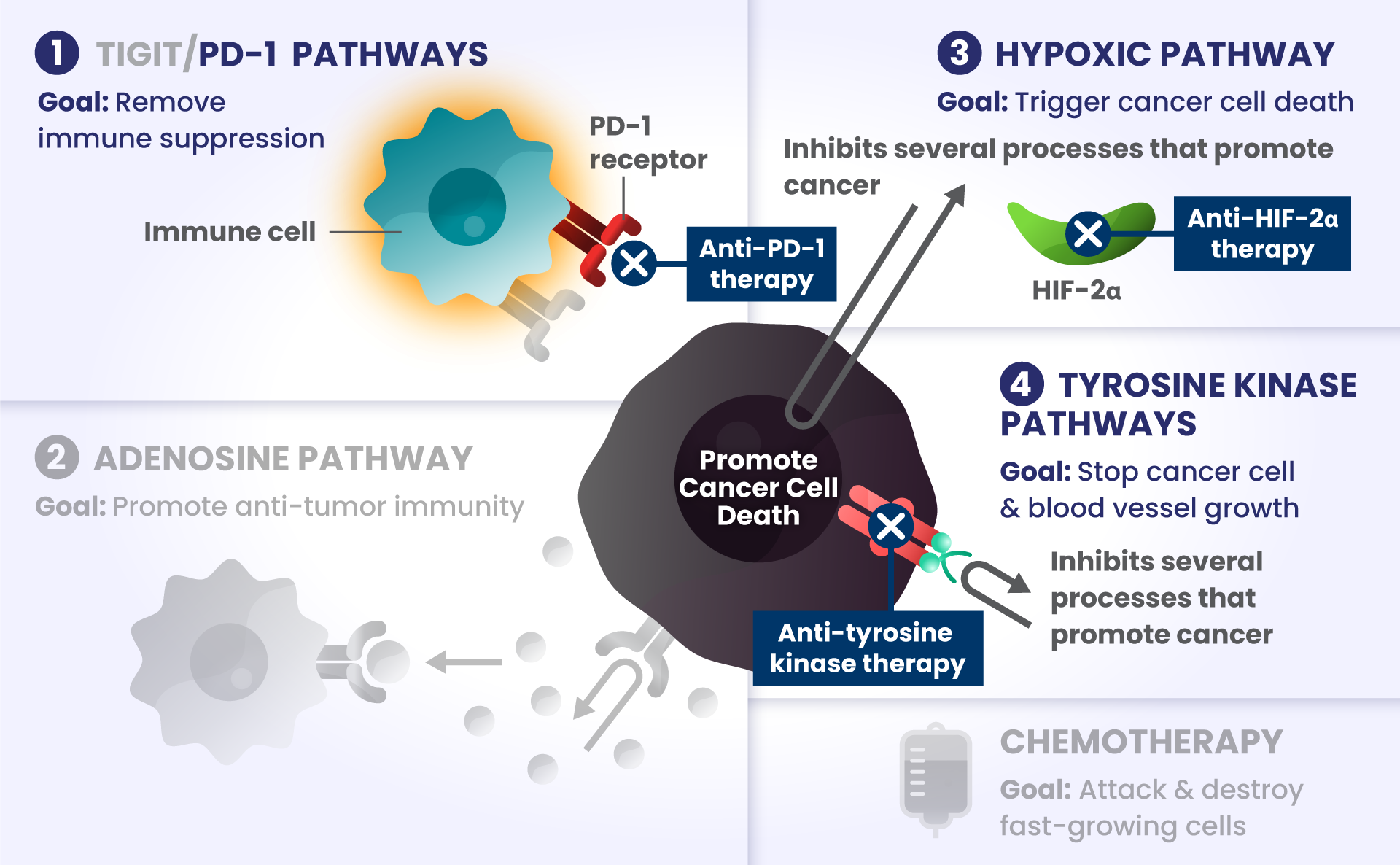
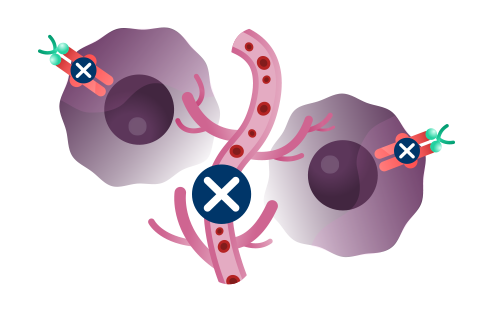
Cancer cells have many ways to stop the immune system from recognizing and destroying them.1 Some of the biological components that may have a role in how the immune system identifies and attacks cancer cells include:
-
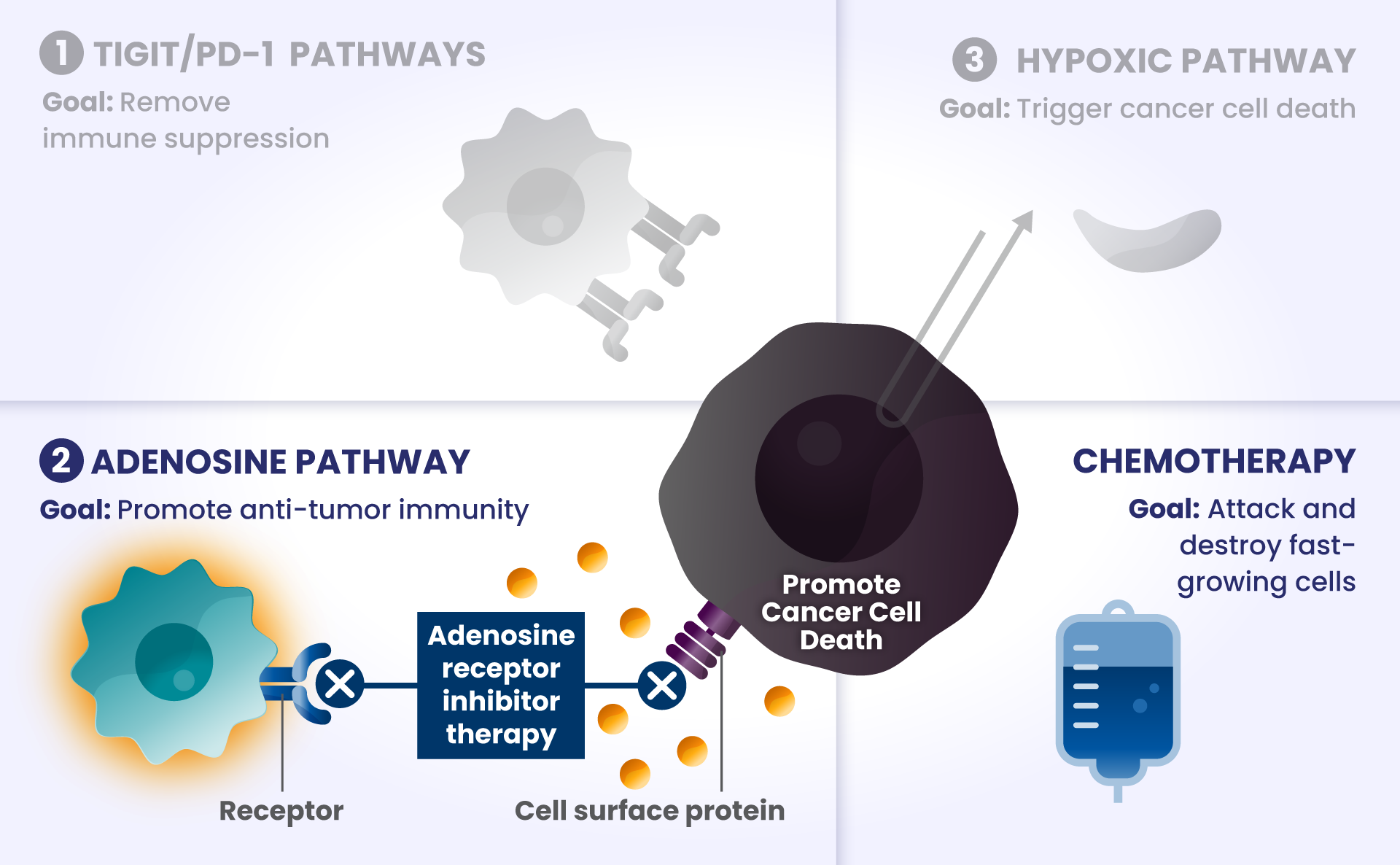
PD-1 and TIGIT: Receptors found at high levels on the surface of certain immune cells in various types of cancer, that are part of a pathway that can block recognition and destruction of cancer cells.1
Imagine cars — as our body’s immune cells — heading towards the Golden Gate Bridge to get to the other side in order to attack and destroy cancer cells.
As they approach the bridge, think of PD-1 and TIGIT like a stop sign and a red traffic light, respectively, that block the cars from crossing the bridge.
-
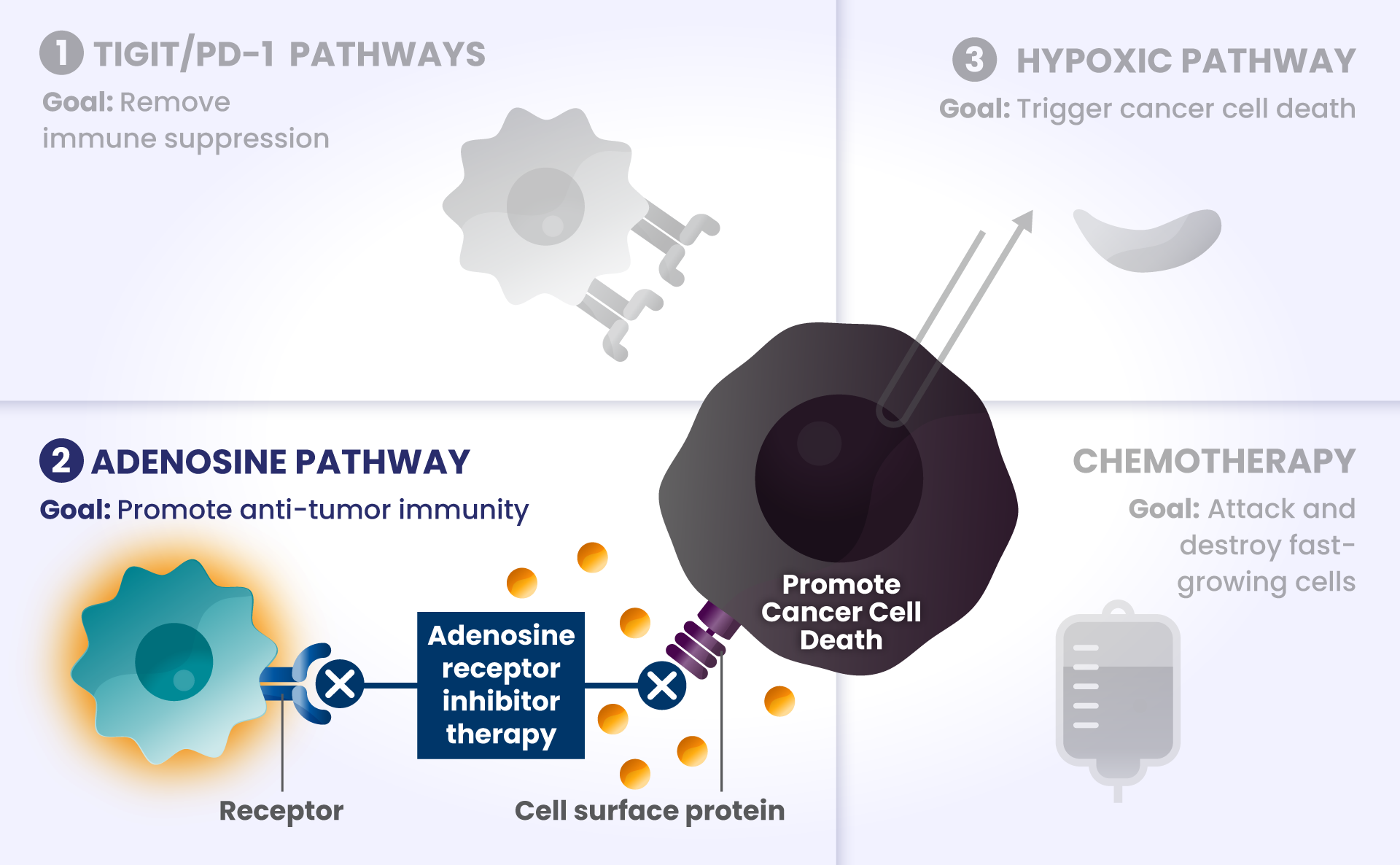
ADENOSINE: A compound that can accumulate in the area surrounding cancer cells, and is made by receptors found at high levels on cancer cells. It binds to other receptors on the surface of immune cells, which deactivates them from attacking cancer cells.2
Picture the cars are now driving on the bridge, but a thick, dense fog – this is like adenosine – rolls in and doesn’t allow the cars to see clearly to get across the bridge as they slowly roll to a stop, preventing them from attacking and destroying the cancer cells on the other side.
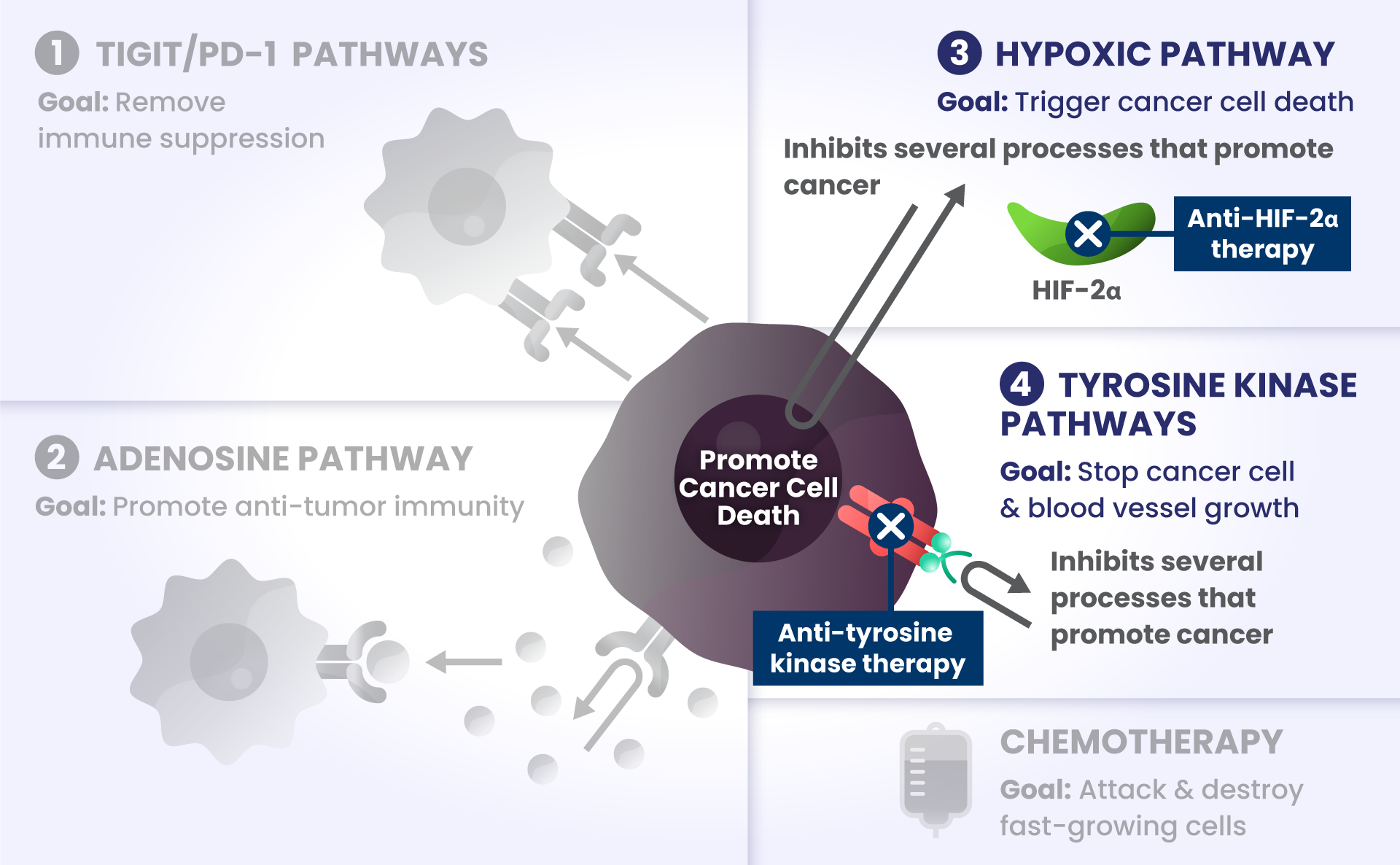
Other biological components are also involved helping cancer cells survive, such as:
-
HIF2α: Tumors often have low oxygen levels, and this regulator can promote growth of tumors in these conditions.3
In many cancers, more than one of these biological components are involved in preventing cancer cell death.4,5,6 Research is underway to determine whether stopping more than one of these biological components at the same time may improve the body’s ability to detect and destroy cancer cells.
Take a closer look at some different cancer types where a biology-driven, combination approach may lead to the development of new treatment approaches and help address unmet needs for people with cancer.
Non-small cell lung cancer
(NSCLC) represents about
Non-small cell lung cancer (NSCLC) represents about
85%
of all lung cancer diagnoses.7
Lung cancer is the:
2nd
most common
type of cancer7*
#1
cause of cancer-
related deaths.7
Current Status of Immunotherapy in NSCLC
Cancer treatment primarily depends on disease stage, which characterizes how big the tumor is and if it has spread throughout the body.8 One treatment approach is immunotherapy, which harnesses the body’s natural immune system to attack and destroy the cancer.1
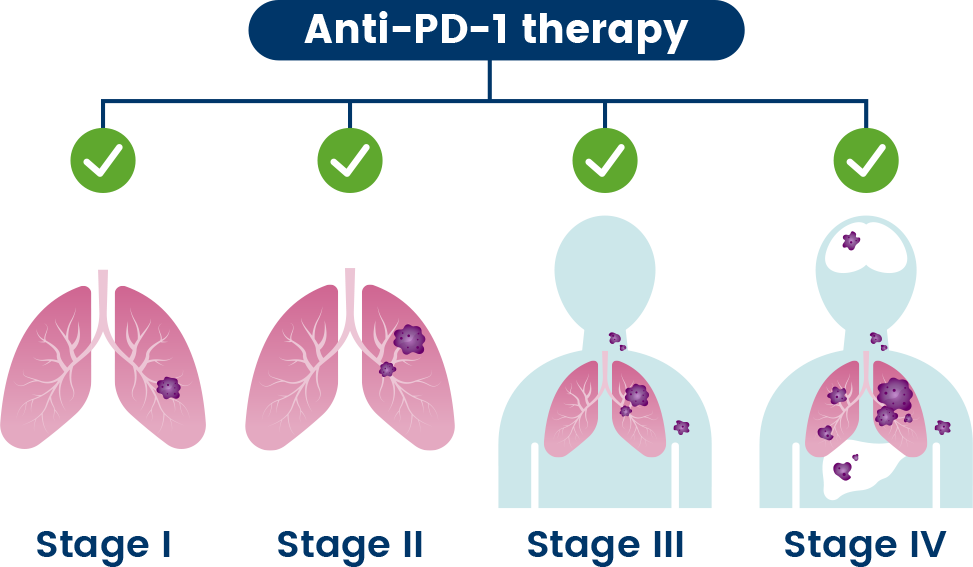
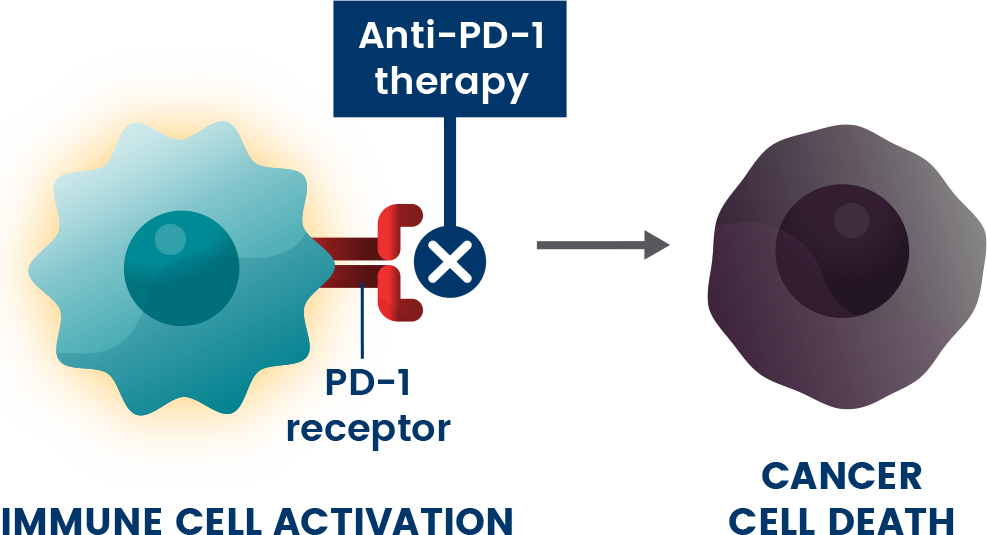
Anti-PD-1s are a type of immunotherapy used to treat different stages of cancer.9 They are believed to work by blocking the PD-1 pathway and activating immune cells to attack cancer cells.1
Combination Strategies Under Investigation
In many people, however, the cancer can continue to grow even with anti-PD-1 treatment.1 Researchers are working to understand if combining multiple investigational medicines, that each target different immune-evading pathways, may lead to the development of new medicines to treat people with cancer.
Clinical trials are ongoing to understand if combining different investigational medicines to stop more than one biological pathway at the same time, with or without chemotherapy, may improve the ability of the immune system to detect and destroy cancer cells and lead to new treatment options.

Colorectal cancer (CRC) is any cancer that starts in the colon or rectum, collectively known as the large intestine.20
According to the American Cancer Society, CRC is the:
3rd
most common diagnosed cancer in the United States*20
2nd
leading cause of cancer-related deaths.20
Current Status of Immunotherapy in CRC
Cancer treatment primarily depends on disease stage, which characterizes how big the tumor is and if it has spread throughout the body.8 One treatment approach is immunotherapy, which harnesses the body’s natural immune system to attack and destroy the cancer.1
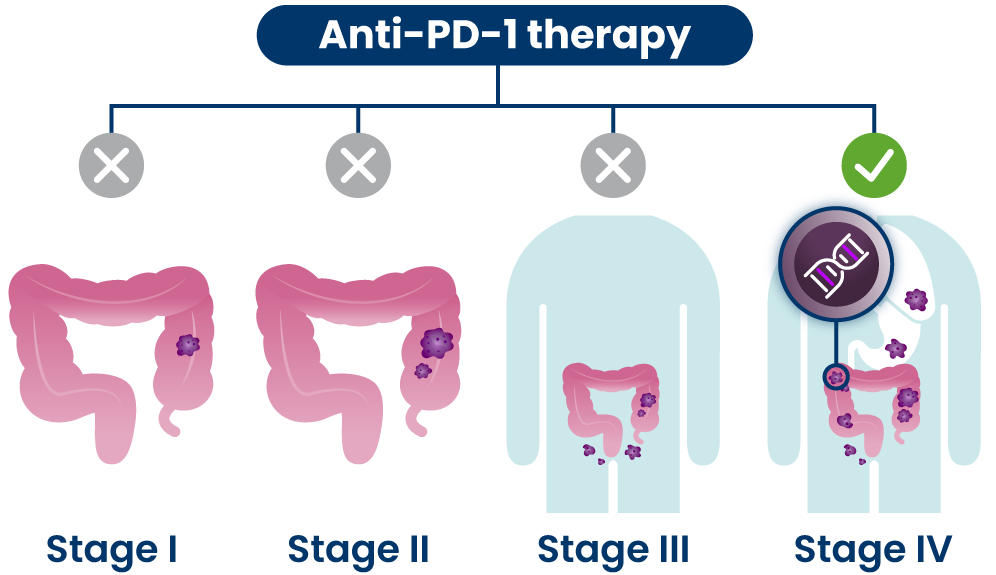
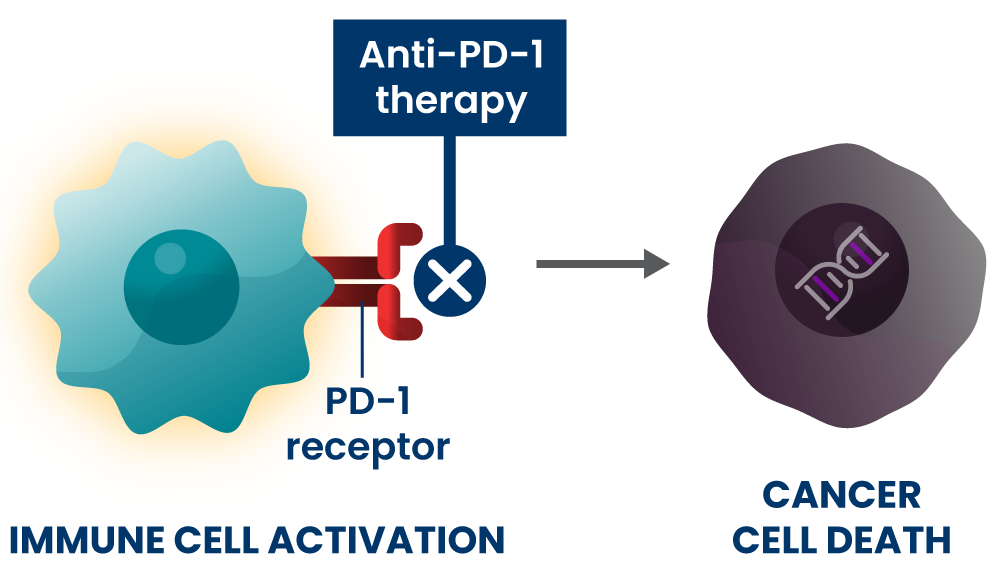
Anti-PD-1s are a type of immunotherapy that are typically used at later stages of disease for people who have certain genetic changes in their cancer cells.21 They are believed to work by blocking the PD-1 pathway and activating immune cells to attack cancer cells.1
Combination Strategies Under Investigation
In many people, however, the cancer can continue to grow even with anti-PD1 treatment.22 Researchers are working to understand if combining anti-PD1 with other investigational medicines that each target different immune-evading pathways may lead to the development of new medicines to treat more people with metastatic cancer, or cancer that has spread to other parts of the body.
Clinical trials are ongoing to understand if combining different investigational medicines to stop more than one biological pathway at the same time, with or without chemotherapy, may improve the ability of the immune system to detect and destroy cancer cells and lead to new treatment options.
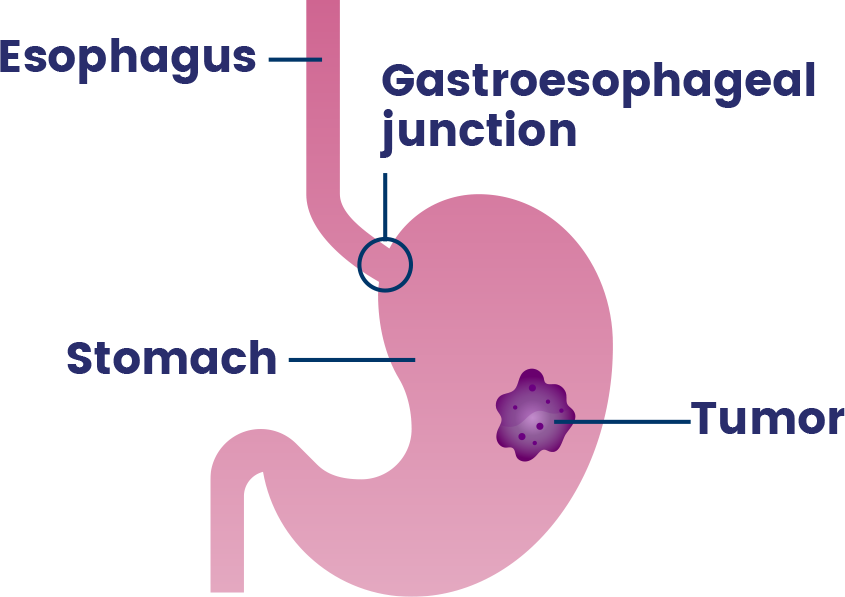
Upper gastrointestinal (GI) cancer includes cancers that occur in the stomach, esophagus (the tube that carries food and drinks from the throat to the stomach) and gastroesophageal junction (where the esophagus connects to the stomach).24
- More than 90% of gastric cancers are adenocarcinomas, a type of cancer that forms in tissues which line certain internal organs and release fluids, like those that help with digestion.25,26
- A majority (up to 80%) of esophageal cancers are adenocarcinomas.27,28
According to the National Cancer Institute, upper GI cancers are the:
2nd
most common cause of death among digestive system cancers.24
They have poor 5-year survival rates when diagnosed in later stages.29
Current Status of Immunotherapy in Upper GI Cancer
Cancer treatment primarily depends on disease stage, which characterizes how big the tumor is and if it has spread throughout the body.8 One treatment approach is immunotherapy, which harnesses the body’s natural immune system to attack and destroy the cancer.1
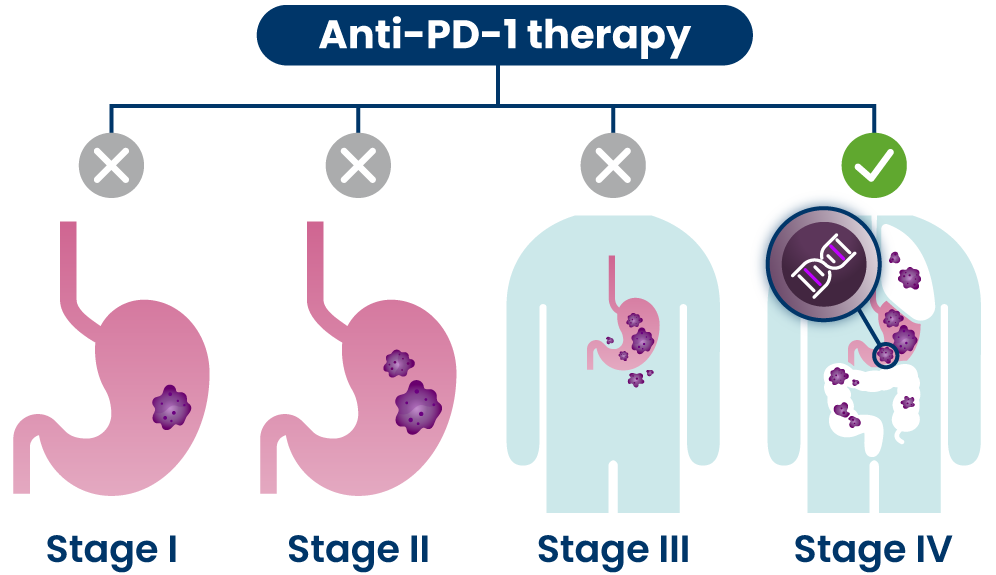

Anti-PD-1s are a type of immunotherapy that are typically used at later stages of disease, including for people who have certain genetic changes in their cancer cells.30,31 They are believed to work by blocking the PD-1 pathway and activating immune cells to attack cancer cells.1
Combination Strategies Under Investigation
In many people, however, the cancer can continue to grow even with anti-PD-1 treatment.32,33 Researchers are working to understand if combining multiple investigational medicines, that each target different immune-evading pathways, may lead to the development of new medicines to treat people with cancer.
Clinical trials are ongoing to understand if combining different investigational medicines to stop more than one biological pathway at the same time, with or without chemotherapy, may improve the ability of the immune system to detect and destroy cancer cells, and lead to new treatment options.
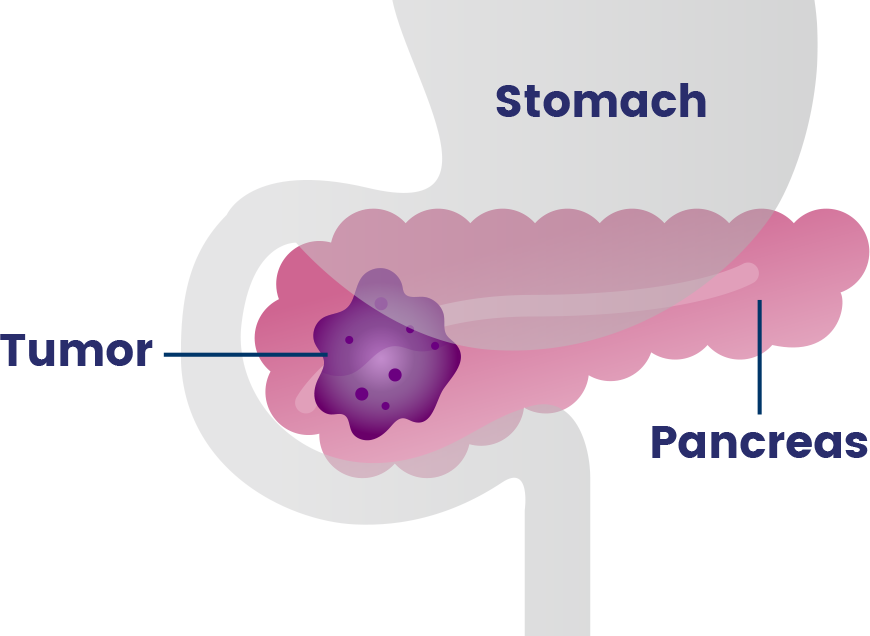
Pancreatic cancer occurs in the pancreas, an organ that sits behind the stomach and helps with digestion and controlling blood sugar.40
- Over 90% of pancreatic cancers are adenocarcinomas, a type of cancer that forms in tissues which line certain internal organs and release fluids, like those that help with digestion.40,41
Pancreatic cancer is one of the most aggressive cancers with an extremely poor prognosis and an average 5-year relative survival rate of
13%42
Over 80% of pancreatic cancers are diagnosed at late stage43
Current Status of Pancreatic Cancer Treatment
Cancer treatment primarily depends on disease stage, which characterizes how big the tumor is and if it has spread throughout the body.8 Some common types of treatments used for advanced pancreatic cancer include:
IMMUNOTHERAPY
Harnessing the body’s natural immune system to detect and destroy cancer.1

CHEMOTHERAPY
A common cancer treatment that attacks fast-growing cells.19

TARGETED THERAPY
Treatments that address specific changes in cancer cells.44
There have been limited advancements for treating pancreatic cancer and chemotherapy has been the standard of care for more than 30 years, which is why new treatment options are needed.45,46,47
Combination Strategies Under Investigation
There are still substantial unmet needs in pancreatic cancer: cancer can come back despite treatment with chemotherapy and there are limitations with immunotherapy as pancreatic tumors are particularly good at hiding from the immune system.48,49 Researchers are working to understand if combining investigational medicines that target immune-evading pathways along with chemotherapy could lead to the development of new therapies to treat more people with metastatic cancer, or cancer that has spread to other parts of the body.
Clinical trials are ongoing to understand if combining different investigational medicines to stop more than one biological pathway at the same time, with or without chemotherapy, may improve the ability of the immune system to detect and destroy cancer cells, and lead to new treatment options.
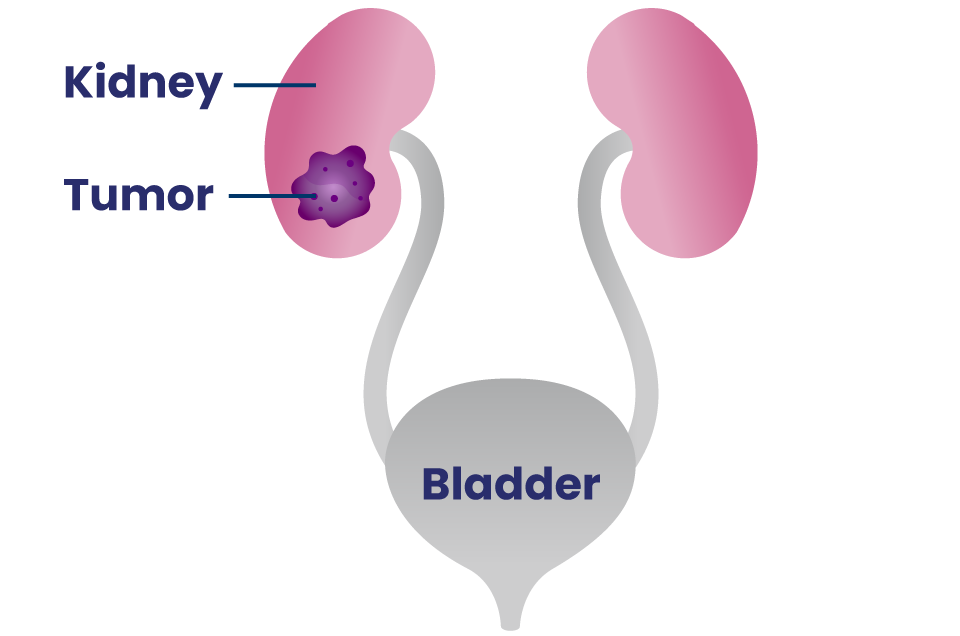
The kidneys are a pair of organs that filter the blood to remove extra water, salt and waste products.58
Renal cell carcinoma (RCC) is by far the most common type of kidney cancer, and clear cell renal cell carcinoma (ccRCC) represents 70% of RCC diagnoses in adults.58
- Carcinomas are cancers that start in cells lining organs or tissues59
- ccRCC is named “clear cell” because when looking at the tumor under a microscope, the cells look pale or clear.58
According to the American Cancer Society, kidney cancer is one of the
10 most common cancers and rates have been rising in recent decades.60
The average 5-year survival rate for those diagnosed with advanced disease is only
17%.61
There remains significant unmet need for patients with ccRCC, because the disease can recur after initial treatment and spread throughout the body.62
Current Status of Kidney Cancer Treatment
Cancer treatment primarily depends on the disease state, which characterizes how big the tumor is and if it has spread throughout the body.13 Some common types of treatments used for advanced kidney cancer include:
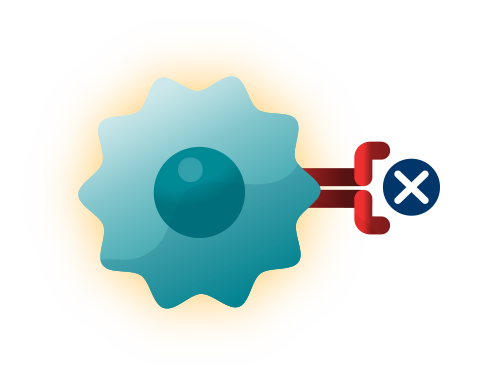
TREATMENTS THAT ADDRESS CHANGES IN AND AROUND CANCER CELLS. For example:63
- Blocking angiogenesis, or the growth of new blood vessels that feed tumors.
- Blocking tyrosine kinases, to prevent excess cell growth and survival. Some tyrosine kinases can also promote angiogenesis, so blocking tyrosine kinases could stop both excessive cell growth and new blood vessel growth.
IMMUNOTHERAPY
Harnessing the body’s natural immune system to detect and destroy cancer.1

CHEMOTHERAPY
A common cancer treatment that attacks fast-growing cells.23,24
Combination Strategies Under Investigation
There is a substantial unmet need for people with the most common form of metastatic kidney cancer, clear cell renal cell carcinoma (ccRCC), which is kidney cancer that has spread to other parts of the body.54 Often, treatments at this stage are used one at a time; but, later stages of kidney cancer can become resistant or stop responding to available approaches.63,65,66,67 Researchers are working to understand if combining multiple investigational medicines, that each address different biological pathways involved in tumor growth, may lead to the development of new treatment approaches for people with metastatic cancer.
Clinical trials are ongoing to understand if combining different investigational medicines to stop more than one biological pathway at the same time may slow or stop cancer growth, and lead to new treatment options.
References:
1. He, Xing, and Chenqi Xu. “Immune checkpoint signaling and cancer immunotherapy.” Cell research. 30.8 (2020): 660-669.
2. Ohta, Akio. “A metabolic immune checkpoint: adenosine in tumor microenvironment.” Frontiers in immunology. 7 (2016): 109.
3. Moreno Roig, Eloy, et al. “Prognostic role of hypoxia-inducible factor-2α tumor cell expression in cancer patients: a meta-analysis.” Frontiers in oncology. 8 (2018): 224.
4. Akinleye, Akintunde, and Zoaib Rasool. “Immune checkpoint inhibitors of PD-L1 as cancer therapeutics.” Journal of hematology & oncology. 12.1 (2019): 92.
5. Ge, Zhouhong, et al. “TIGIT, the next step towards successful combination immune checkpoint therapy in cancer.” Frontiers in Immunology. 12 (2021): 699895.
6. Zhao, J., et al. “The role of hypoxia-inducible factor-2 in digestive system cancers.” Cell death & disease. 6.1 (2015): e1600-e1600.
7. “About Lung Cancer.” American Cancer Society, February 2024 & January 2025. https://www.cancer.org/cancer/lung-cancer/about.html
8. “Cancer Staging.” National Cancer Institute. October 2022. https://www.cancer.gov/about-cancer/diagnosis-staging/staging
9. “Treating Non-Small Cell Lung Cancer.” American Cancer Society, January 2023. https://www.cancer.org/cancer/lung-cancer/treating-non-small-cell.html
10. Sun, Yu, et al. “Combined evaluation of the expression status of CD155 and TIGIT plays an important role in the prognosis of LUAD (lung adenocarcinoma).” International immunopharmacology. 80 (2020): 106198.
11. Pawelczyk, Konrad, et al. “Role of PD-L1 expression in non-small cell lung cancer and their prognostic significance according to clinicopathological factors and diagnostic markers.” International journal of molecular sciences. 20.4 (2019): 824.
12. Banta, Karl L., et al. “Mechanistic convergence of the TIGIT and PD-1 inhibitory pathways necessitates co-blockade to optimize anti-tumor CD8+ T cell responses.” Immunity. 55.3 (2022): 512-526.
13. Guan, Shuxiao, et al. “Metabolic reprogramming by adenosine antagonism and implications in non-small cell lung cancer therapy.” Neoplasia. 32 (2022): 100824.
14. Sun, Changfa, Bochu Wang, and Shilei Hao. “Adenosine-A2A receptor pathway in cancer immunotherapy.” Frontiers in Immunology. (2022): 1195.
15. Chen, Siqi, et al. “The Expression of Adenosine A2B Receptor on Antigen-Presenting Cells Suppresses CD8+ T-cell Responses and Promotes Tumor GrowthAPC Expression of Adenosine A2BR Controls Tumor Growth.” Cancer immunology research. 8.8 (2020): 1064-1074.
16. Fares, Charlene M., et al. “Mechanisms of resistance to immune checkpoint blockade: why does checkpoint inhibitor immunotherapy not work for all patients?” American Society of Clinical Oncology Educational Book. 39 (2019): 147-164.
17. Leone, Robert D., Ying-Chun Lo, and Jonathan D. Powell. “A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy.” Computational and structural biotechnology journal. 13 (2015): 265-272.
18. “How is Chemotherapy Used to Treat Cancer?” American Cancer Society, November 2019. https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/chemotherapy/how-is-chemotherapy-used-to-treat-cancer.html
19. “How Chemotherapy Drugs Work.” American Cancer Society, November 2019. https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/chemotherapy/how-chemotherapy-drugs-work.html
20. “About Colorectal Cancer.” American Cancer Society, January 2024 & January 2025. https://www.cancer.org/cancer/colon-rectal-cancer/about.html
21. “Treating Colorectal Cancer.” American Cancer Society, June 2020. https://www.cancer.org/cancer/colon-rectal-cancer/treating.html
22. Makaremi, Shima, et al. “Immune checkpoint inhibitors in colorectal cancer: challenges and future prospects.” Biomedicines. 9.9 (2021): 1075.
23. Hajizadeh, Farnaz, et al. “Adenosine and adenosine receptors in colorectal cancer.” International immunopharmacology. 87 (2020): 106853.
24. “Introduction to UGI Cancer.” National Cancer Institute. https://training.seer.cancer.gov/ugi/intro/
25. “What is Stomach Cancer?” American Cancer Society. https://www.cancer.org/cancer/types/stomach-cancer/about/what-is-stomach-cancer.html.
26. “Adenocarcinoma” National Cancer Institute. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/adenocarcinoma.
27. “Esophageal Cancer” National Cancer Institute. https://www.cancer.gov/pediatric-adult-rare-tumor/rare-tumors/rare-digestive-system-tumors/esophageal.
28. Then, Eric Omar, et al. “Esophageal Cancer: An Updated Surveillance Epidemiology and End Results Database Analysis.” World Journal of Oncology. 11 (2020): 55-64.
29. “Five-Year Survival Rates.” National Cancer Institute. https://training.seer.cancer.gov/ugi/intro/survival.html.
30. “Immunotherapy for Stomach Cancer.” American Cancer Society, February 2022. https://www.cancer.org/cancer/types/stomach-cancer/treating/immunotherapy.html.
31. “Immunotherapy for Esophageal Cancer.” American Cancer Society, June 2022. https://www.cancer.org/cancer/types/esophagus-cancer/treating/immunotherapy.html.
32. Cheng, Chao, et al. “Overcoming resistance to PD-1/PD-L1 inhibitors in esophageal cancer.” Frontiers in Oncology. 12 (2022): 955163.
33. Voutsadakis, Ioannis A. “A systematic review and meta-analysis of PD-1 and PD-L1 inhibitors monotherapy in metastatic gastric and gastroesophageal junction adenocarcinoma.” Euroasian journal of hepato-gastroenterology. 10.2 (2020): 56.
34. Wang, Daijun, et al. “Role of CD155/TIGIT in digestive cancers: promising cancer target for immunotherapy.” Frontiers in Oncology. 12 (2022).
35. Xie, Jinhua, et al. “Expression of immune checkpoints in T cells of esophageal cancer patients.” Oncotarget. 7.39 (2016): 63669.
36. Kono, Yusuke, et al. “Increased PD-1-positive macrophages in the tissue of gastric cancer are closely associated with poor prognosis in gastric cancer patients.” BMC cancer. 20.1 (2020): 1-9.
37. Wang, Peipei, et al. “Increased coexpression of PD-L1 and TIM3/TIGIT is associated with poor overall survival of patients with esophageal squamous cell carcinoma.”Journal for Immunotherapy of Cancer. 9.10 (2021).
38. Wang, Junqing, Linyong Du, and Xiangjian Chen. “Adenosine signaling: Optimal target for gastric cancer immunotherapy.” Frontiers in Immunology. 13 (2022).
39. Chen, Yen-Hao, et al. “CD73 promotes tumor progression in patients with esophageal squamous cell carcinoma.” Cancers. 13.16 (2021): 3982.
40. “What is Pancreatic Cancer?” American Cancer Society, January 2025. https://www.cancer.org/cancer/types/pancreatic-cancer/about/what-is-pancreatic-cancer.html.
41. Sarantis, Panagiotis, et al. “Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy.” World journal of gastrointestinal oncology. 12.2 (2020): 173.
42. “Survival Rates for Pancreatic Cancer.” American Cancer Society. January 2025. https://www.cancer.org/cancer/types/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html.
43. Gallegos, Jillian M., et al. “Socioeconomic Factors Associated With a Late-Stage Pancreatic Cancer Diagnosis: An Analysis of the National Cancer Database.” Cureus 15.3 (2023).
44. “Targeted Therapy for Pancreatic Cancer.” American Cancer Society, December 2024. https://www.cancer.org/cancer/types/pancreatic-cancer/treating/targeted-therapy.html.
45. Roth, Marc T., Dana B. Cardin, and Jordan D. Berlin. “Recent advances in the treatment of pancreatic cancer.” F1000Research. 9 (2020).
46. Brown, Timothy J., Kim A. Reiss, and Mark H. O’Hara. “Advancements in Systemic Therapy for Pancreatic Cancer.” American Society of Clinical Oncology Educational Book. 43 (2023): e397082.
47. Mohammed, Somala, I. I. George Van Buren, and William E. Fisher. “Pancreatic cancer: advances in treatment.” World journal of gastroenterology: WJG. 20.28 (2014): 9354.
48. Adel, Nelly. “Current Treatment Landscape and Emerging Therapies for Pancreatic Cancer.” American Journal of Managed Care. 25.1 (2019).
49. Netto, Daniel, et al. “Systemic Therapy for Metastatic Pancreatic Cancer—Current Landscape and Future Directions.” Current Oncology. 31.9 (2024): 5206–5223.
50. Faraoni, Erika Y., et al. “CD73-dependent adenosine signaling through Adora2b drives immunosuppression in ductal pancreatic cancer.” Cancer Research. 83.7 (2023): 1111-1127.
51. Graziano, Vincenzo, et al. “Defining the spatial distribution of extracellular adenosine revealed a myeloid-dependent immunosuppressive microenvironment in pancreatic ductal adenocarcinoma.” Journal for Immunotherapy of Cancer. 11.8 (2023).
RECENT ARTICLES
Combining for Change in Kidney Cancer
While there has been substantial progress in treatment in recent years, even more innovative approaches are needed to improve survival, especially when the cancer is metastatic, meaning it has spread to other parts of the body.
From Paper to Pill
There is opportunity to develop new and potentially improved molecules that block HIF-2α to further improve outcomes.