Taking the Brakes Off the Immune System: Exploring Immunotherapy Combinations
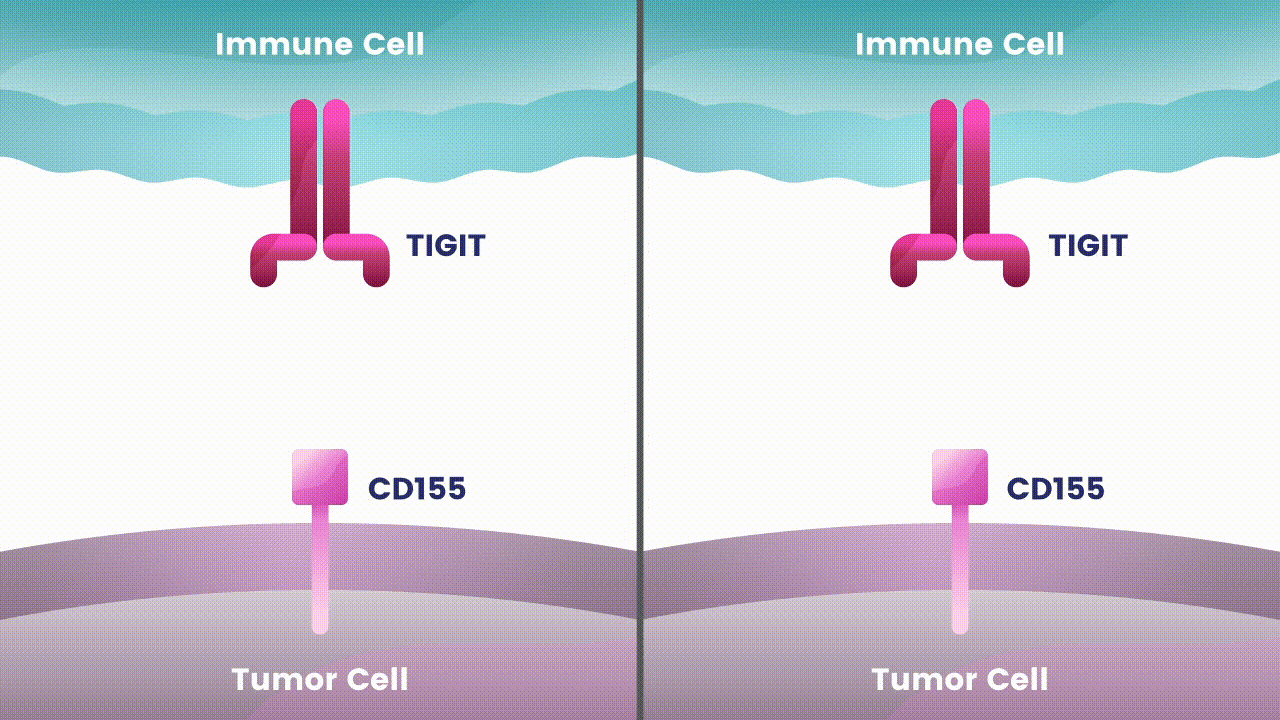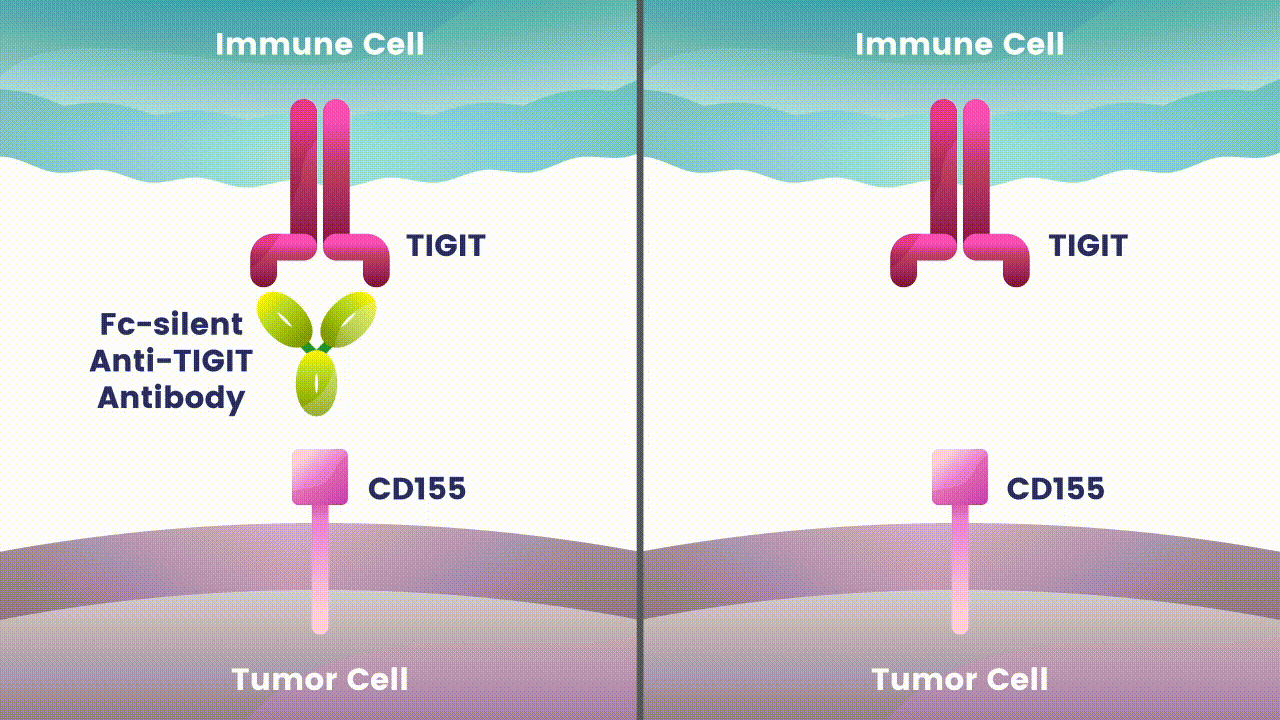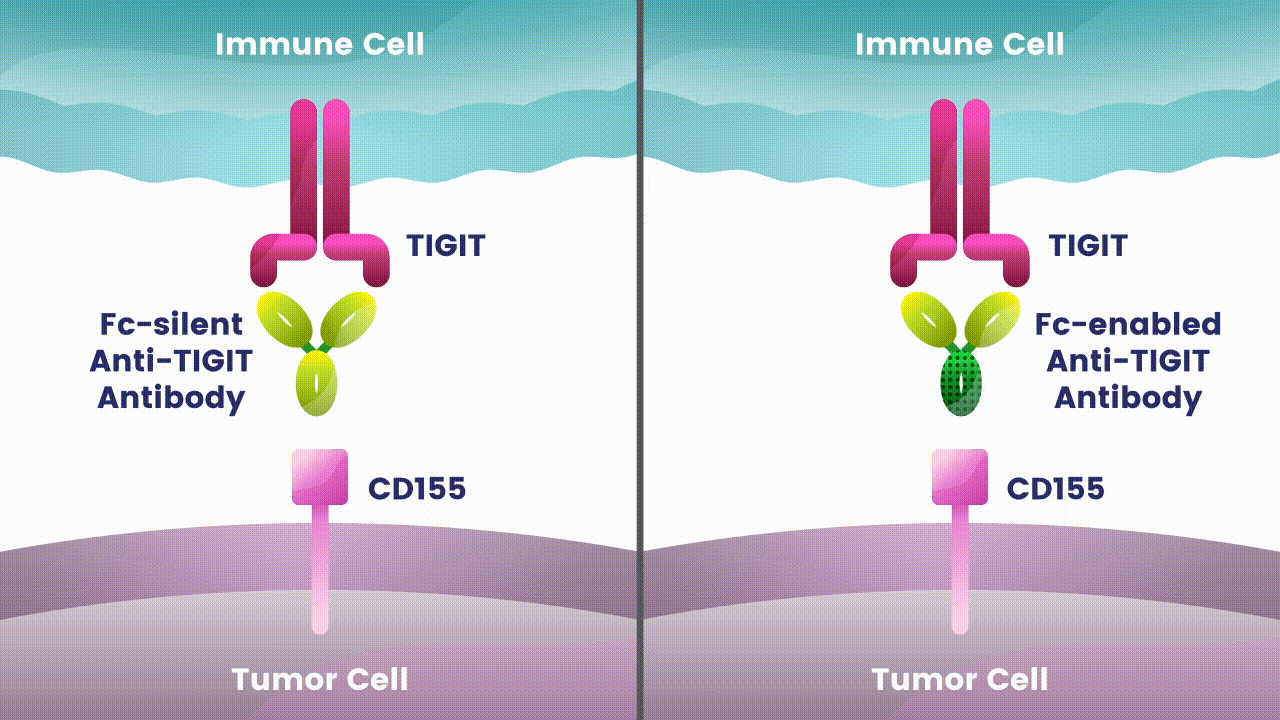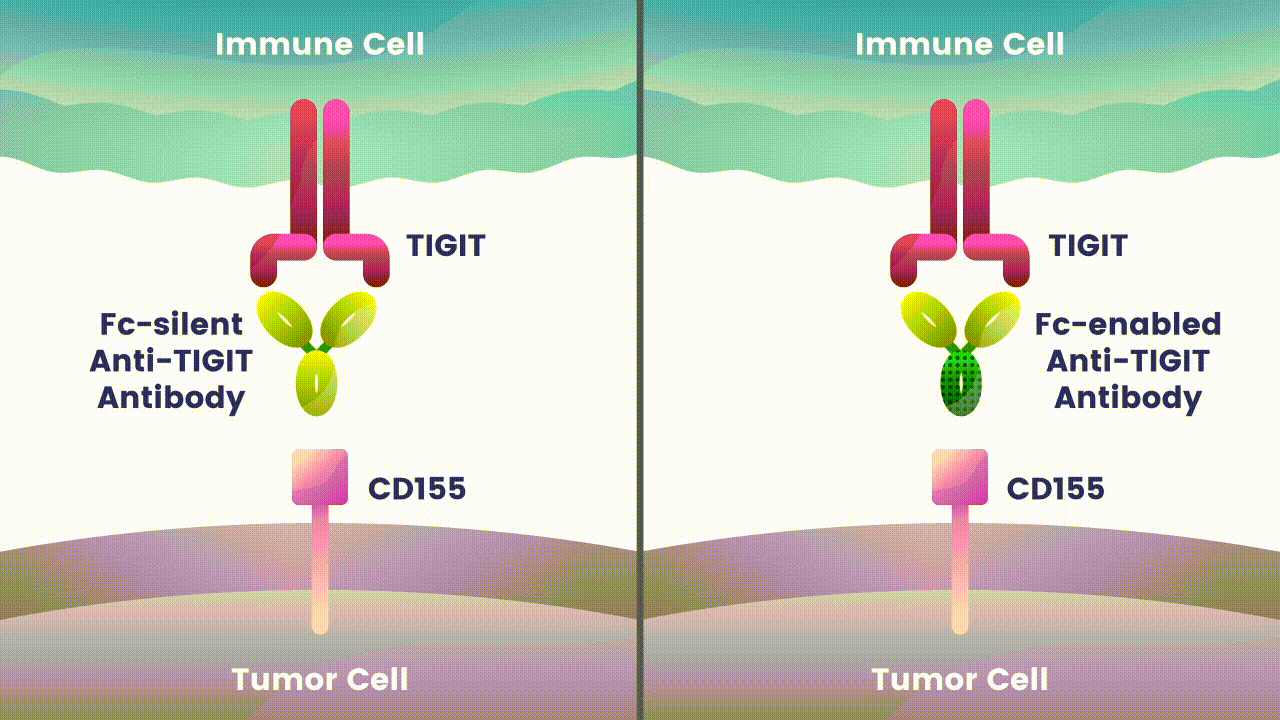
Our immune system is made up of different types of cells that search for and destroy foreign invaders. These adversaries can include bacteria and viruses, as well as abnormal versions of our own cells, like those in cancerous tumors. However, tumor cells have developed many strategies to evade the body’s natural defenses.1 One is to deactivate immune cells that recognize and attack tumor cells.1 Many tumor cells carry proteins on their surface that tell our immune cells not to attack.1 These proteins are called immune checkpoints, and under normal circumstances they keep the immune system from attacking healthy cells in our body.1 Tumor cells use these checkpoints to stop immune cells from finding and eliminating them, like applying an emergency brake at the wrong time.
Cancer immunotherapy, which harnesses the body’s natural immune defenses, has transformed treatment for some patients with difficult-to-treat types of cancer.1 Immune checkpoint inhibitors, a type of cancer immunotherapy, block the signals tumor cells use to escape the immune system’s defenses.1
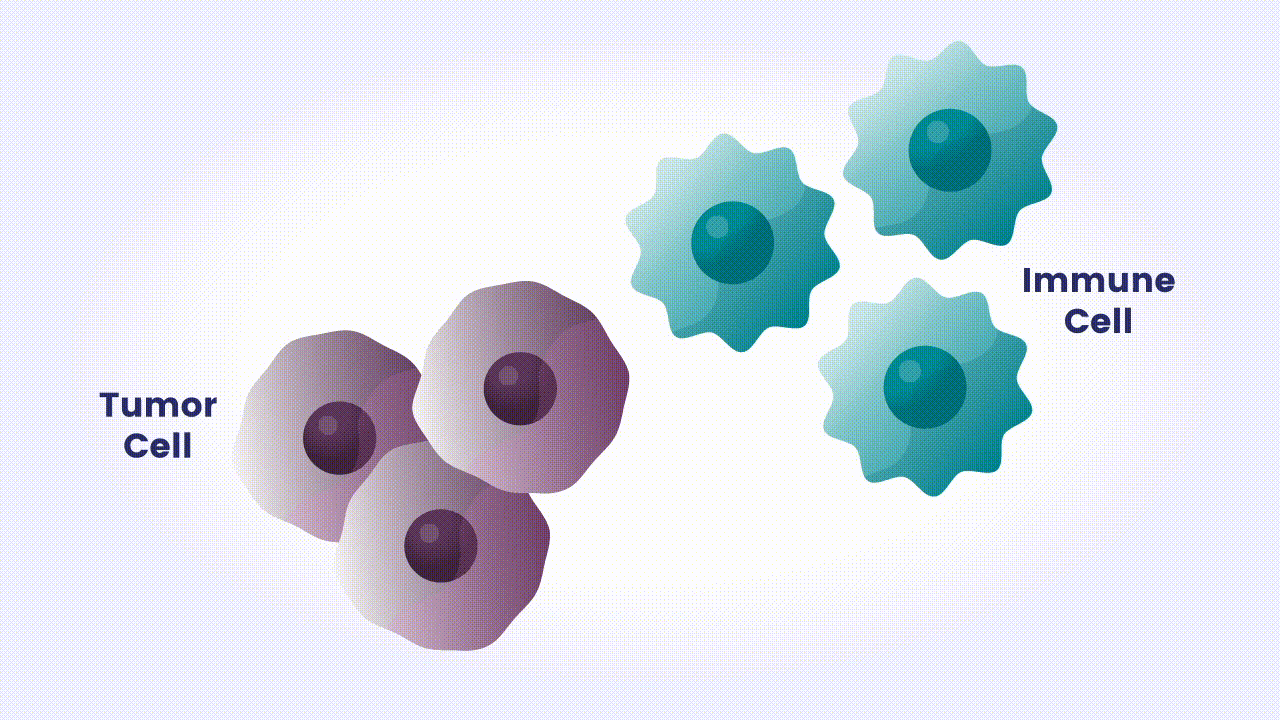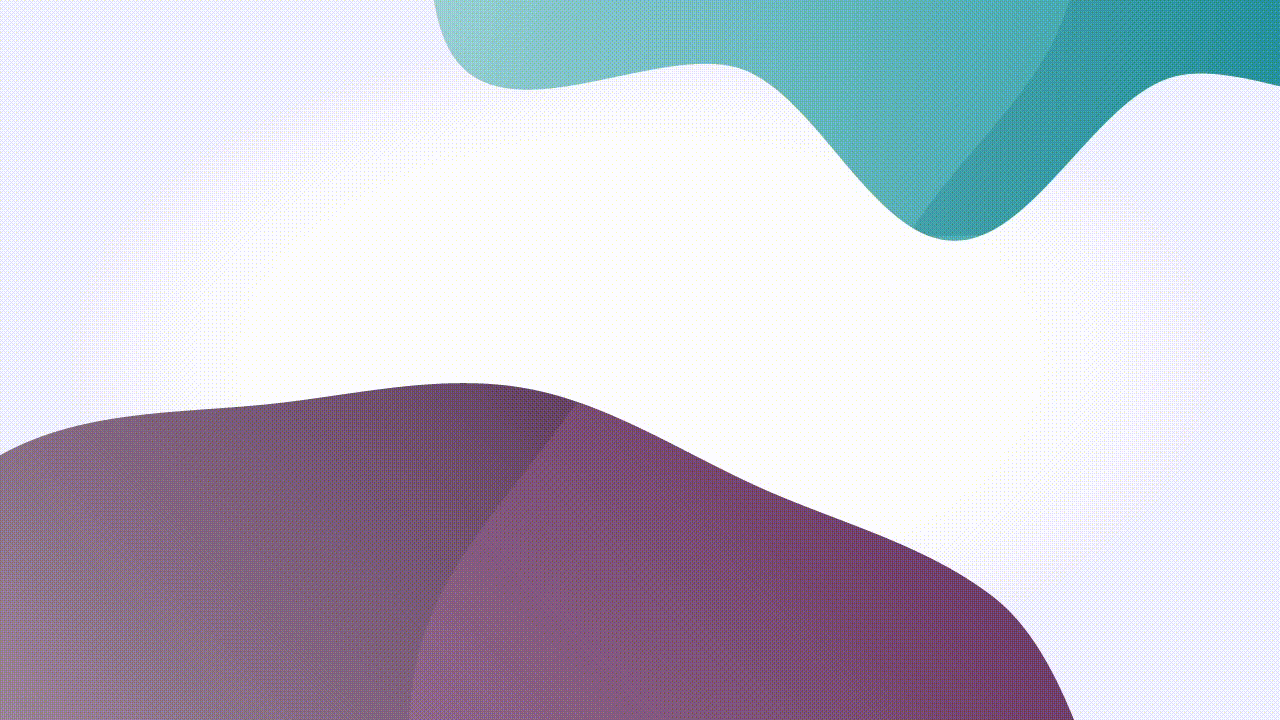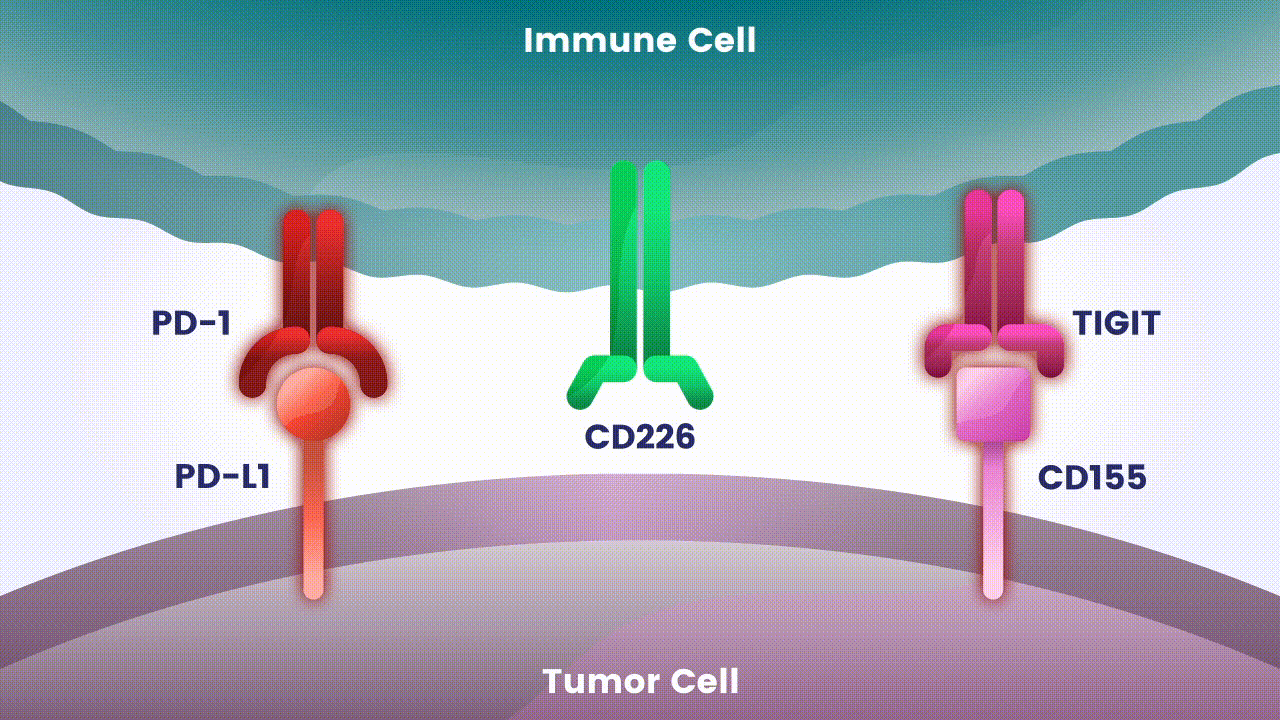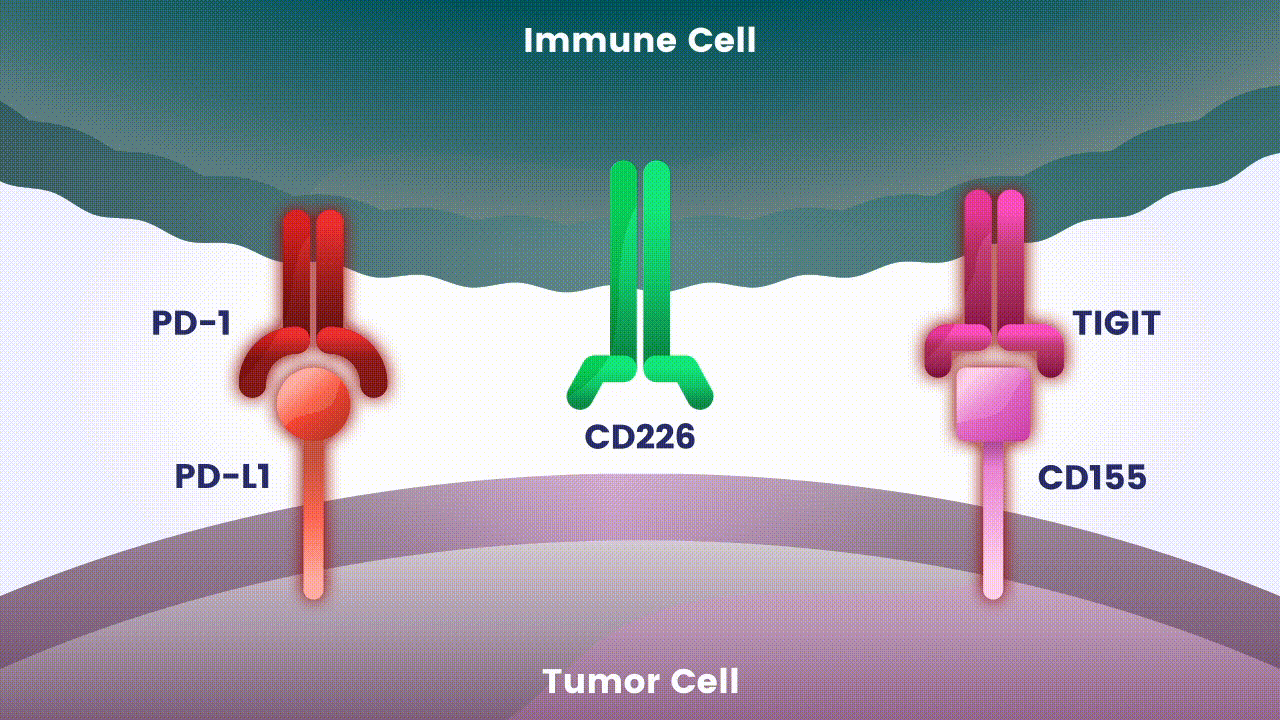
Disrupting more than one checkpoint is one approach to potentially change the effectiveness of cancer immunotherapy.2 Let’s take a closer look at two specific immune checkpoints Arcus scientists are studying as an investigational combination treatment for certain types of cancer.
Preliminary studies have provided a scientific rationale for continuing research on the effects of combining anti-PD-1 antibodies with anti-TIGIT antibodies, since these checkpoints appear to play distinct, but potentially complementary roles in modulating anti-tumor responses.2 However, the effects of the various approaches to targeting TIGIT are still unknown.
Research at Arcus is underway to evaluate Fc-silent anti-TIGIT and PD-(L)-1 antibody combinations in cancers such as lung and gastrointestinal. While available checkpoint inhibitors are currently approved for use in both cancer types, their effectiveness varies in different patients.6,7,8 Studies show that TIGIT is present at high levels in non-small cell lung cancer and upper gastrointestinal tract cancers and can contribute to tumors coming back.9,10 TIGIT is also thought to impact a broad range of immune cells.3 Arcus is studying the effect of targeting TIGIT in combination with existing checkpoint blockers, like PD-(L)-1 inhibitors, particularly in cancers with high unmet need. This could both release the brake and push the accelerator on immune response to cancer.
In the future, Arcus plans to evaluate the combination of immune checkpoint inhibition with novel approaches to overcoming immune cell deactivation. One approach under investigation is blocking the effects of adenosine. Within healthy cells, adenosine has a wide variety of functions, but excess amounts of adenosine can accumulate outside tumor cells and deactivate immune cells.11 Based on preliminary studies, one area of interest is inhibiting adenosine along with immune checkpoints.12 Our goal at Arcus is to refine immune checkpoint-based therapy by simultaneously targeting key pathways with the potential to reverse immune cell suppression and destroy tumor cells.
References:
1. He, Xing, and Chenqi Xu. “Immune checkpoint signaling and cancer immunotherapy.” Cell research 30.8 (2020): 660-669.
2. Banta, Karl L., et al. “Mechanistic convergence of the TIGIT and PD-1 inhibitory pathways necessitates co-blockade to optimize anti-tumor CD8+ T cell responses.” Immunity 55.3 (2022): 512-526.
3. Chauvin, Joe-Marc, and Hassane M. Zarour. “TIGIT in cancer immunotherapy.” Journal for immunotherapy of cancer 8.2 (2020).
4. Gogesch, Patricia, et al. “The role of FC receptors on the effectiveness of therapeutic monoclonal antibodies.” International Journal of Molecular Sciences 22.16 (2021): 8947.
5. Rotte, Anand, Srikumar Sahasranaman, and Nageshwar Budha. “Targeting TIGIT for immunotherapy of cancer: Update on clinical development.” Biomedicines 9.9 (2021): 1277.
6. Zimmermann, Stefan, et al. “Immune checkpoint inhibitors in the management of lung cancer.” American Society of Clinical Oncology Educational Book 38 (2018): 682-695.
7. Baxter, Mark A., et al. “Resistance to immune checkpoint inhibitors in advanced gastro-oesophageal cancers.” British Journal of Cancer 125.8 (2021): 1068-1079.
8. Schoenfeld, Adam J., and Matthew D. Hellmann. “Acquired resistance to immune checkpoint inhibitors.” Cancer cell 37.4 (2020): 443-455.
9. Sun, Yu, et al. “Combined evaluation of the expression status of CD155 and TIGIT plays an important role in the prognosis of LUAD (lung adenocarcinoma).” International immunopharmacology 80 (2020): 106198.
10. Wang, Daijun, et al. “Role of CD155/TIGIT in Digestive Cancers: Promising Cancer Target for Immunotherapy.” Frontiers in Oncology 12 (2022).
11. Ohta, Akio. “A metabolic immune checkpoint: adenosine in tumor microenvironment.” Frontiers in immunology 7 (2016): 109.
12. Piovesan, Dana, et al. “Targeting CD73 with AB680 (Quemliclustat), a novel and potent small molecule CD73 inhibitor, restores immune functionality and facilitates anti-tumor immunity.” Molecular Cancer Therapeutics (2022).
RECENT ARTICLES
Combining for Change in Kidney Cancer
While there has been substantial progress in treatment in recent years, even more innovative approaches are needed to improve survival, especially when the cancer is metastatic, meaning it has spread to other parts of the body.
From Paper to Pill
There is opportunity to develop new and potentially improved molecules that block HIF-2α to further improve outcomes.