





A Peek Into the Platform: How an Innovative Trial Design Can Accelerate Drug Development
We are living in the golden age of biotechnology. Thanks to significant advances in science, technology and data, we now have access to massive amounts of information that can help the biopharma industry develop new treatments that help more patients.
Virtually every day we gain new insights into diseases like cancer — from pathways that drive tumor growth to mechanisms cancer cells use to evade the immune system. At the same time, we are evaluating more novel treatment approaches than ever before.
To evaluate the growing number of treatment options efficiently and rigorously, we need to rethink the way we approach early clinical trials so we can cut through the noise and focus on the most promising potential treatments for patients. We can no longer settle for asking one question at a time.
Seeking Signals
The potential treatments in Arcus’ pipeline are designed to be highly combinable, in pursuit of the most impactful combinations that may eventually provide cures for patients living with cancer. As one can imagine, the possibilities for combining treatments are vast, especially given that we are combining our investigational therapies with each other, as well as with any number of existing treatments, such as chemotherapy, immunotherapies and targeted therapies. To find the most promising combinations quickly, we are deploying an innovative trial design known as a platform clinical trial.
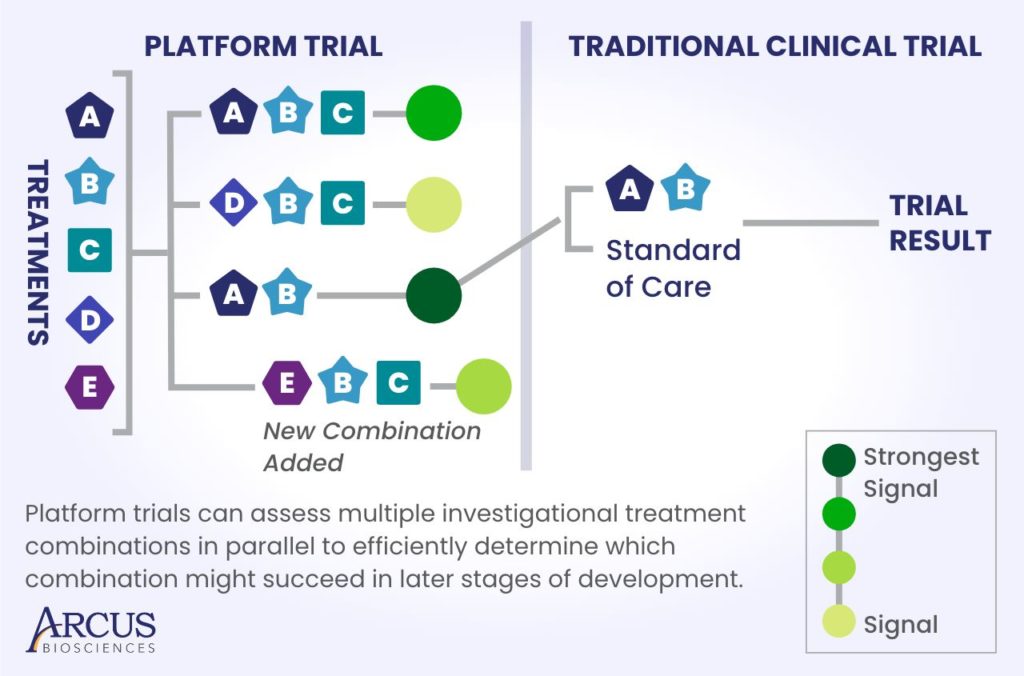
Platform clinical trials do not replace traditional clinical trials, which remain the gold standard for measuring how well a treatment works and whether it is safe. However, they do allow us to quickly and efficiently determine which combinations will have the best chance of succeeding in traditional clinical trials at later stages of development.
While randomized controlled trials are designed to measure the impact of one variable – such as the effect of an experimental treatment compared to standard of care – in a patient population, platform trials can ask many questions simultaneously. For example, researchers could use a platform trial to evaluate multiple combinations of treatments, in multiple lines of therapy, testing each approach in small groups of patients.
This approach allows us to look for signals of efficacy and safety across many novel combinations in a relatively short time. When we design platform clinical trials, we expect that some arms will not move forward. This doesn’t mean those combinations “failed.” Rather, each preliminary readout from each arm of the study serves as a piece of a larger puzzle that helps us narrow our candidates down to the most promising combinations or inform which combinations we should evaluate next.
Accelerating the Path from Signal to Treatment
Platform trials do not reveal anything we could not learn through traditional clinical trials, assuming we had unlimited time and resources to study every possible combination of novel treatments one at a time. And there lies the utility of platform studies. If we can reduce the time it takes to develop lifesaving treatments, we must try. Because patients are waiting. Besides screening multiple combination treatments at once, platform studies allow us to add new treatment arms along the way, which can save time and cost compared to designing and conducting an entirely new trial. These innovative trials also save human resources, since they require smaller teams and fewer trial sites than separate traditional trials would.
Our pipeline at Arcus is a testament to the power of this approach. After just seven years of operations, we have several potential treatment combinations in mid- and late-stage studies, supported by data generated in earlier phase signal‑seeking studies.
We hope more peers in the biopharma industry embrace platform trials – with the support of regulatory agencies around the world – to rapidly translate the unprecedented wealth of data at our disposal into more lifesaving treatments for patients.
RECENT ARTICLES
Combining for Change in Kidney Cancer
While there has been substantial progress in treatment in recent years, even more innovative approaches are needed to improve survival, especially when the cancer is metastatic, meaning it has spread to other parts of the body.
From Paper to Pill
There is opportunity to develop new and potentially improved molecules that block HIF-2α to further improve outcomes.

