





The Fc-silent Treatment: Pursuing a Known Target in a New Way
The biological interactions between cancer and immune cells are complex across tumor types. In some cases, multiple treatments with different functions or targets can be combined to provide options that address this underlying complexity. We are developing novel immunotherapy treatment combinations that harness the body’s natural immune defenses to kill cancer cells.
TIGIT has emerged as a promising new immune checkpoint to inhibit because it is expressed on immune cells in several types of cancer.1 TIGIT can deactivate immune cells and prevent them from recognizing and killing cancer cells.2
Antibodies used to target TIGIT are designed in different ways, with their Fc region (the part of the antibody that faces away from TIGIT) either “enabled” (able to interact with various Fc receptors) or “silent” (mutated to prevent interaction with the Fc receptors). The vast majority of anti-TIGIT antibodies in clinical development are Fc-enabled, meaning they can activate immune processes that may eliminate cancer-killing immune cells. Until recently, there were no clinical data to support whether Fc activity is needed for clinical benefit. Yet, the hypothesis that Fc activity is required was the prevailing belief in the field.
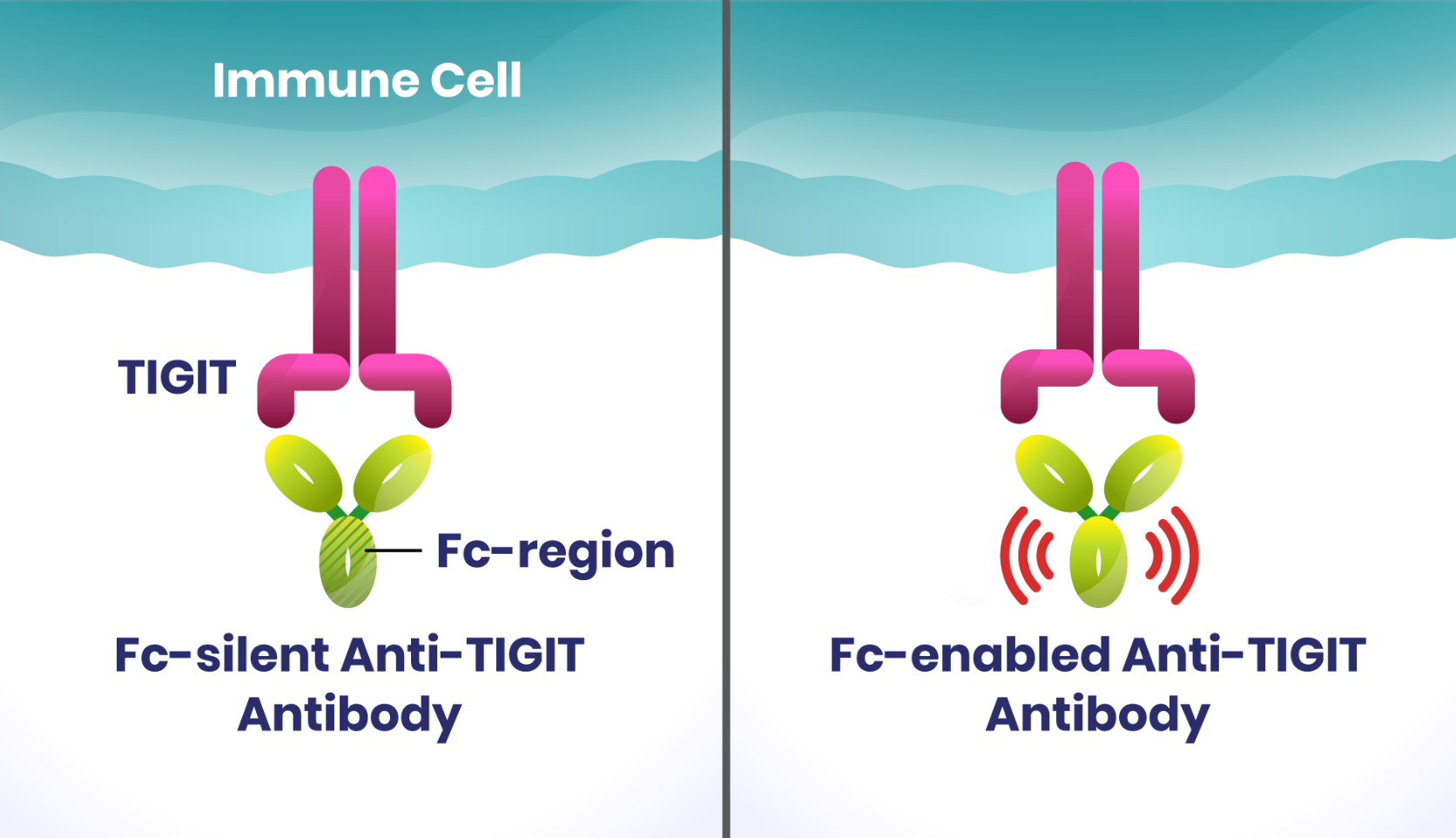
We were among the first to take a different approach and pursue an Fc-silent anti-TIGIT antibody based on our own scientific understanding of how this biological pathway works. Both Fc-silent and Fc-enabled antibodies can block TIGIT’s inhibition of the immune system, but unlike Fc-enabled antibodies, an Fc-silent one may also reduce the possibility of killing two types of cells: TIGIT-expressing immune cells that are capable of killing cancer cells, and regulatory T cells (Treg) outside the tumor where they control unwanted immune responses against healthy tissue.3
Designed to be Combined
Since blocking TIGIT alone may not promote full activation of immune cells, we are studying an investigational Fc-silent anti-TIGIT antibody (domvanalimab) specifically designed to be used in combination with other treatments. Our intention is to combine the Fc-silent antibody with other immunotherapy options without compounding the potential harmful side effects often associated with multi-treatment regimens.
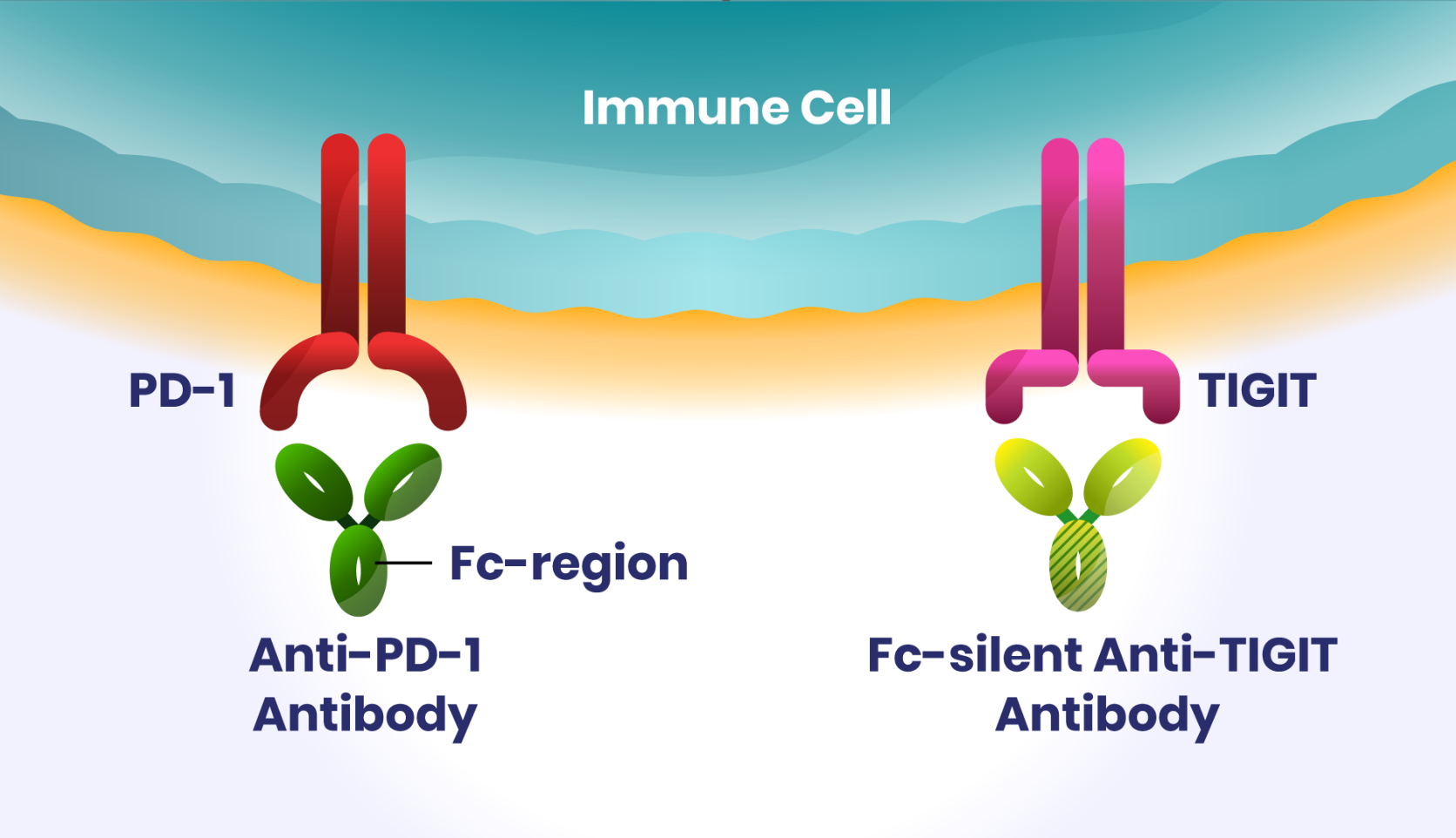
Domvanalimab is the most clinically advanced investigational Fc-silent anti-TIGIT antibody in development and is the first of its kind to move into pivotal Phase III studies. In preclinical studies, domvanalimab in combination with investigational zimberelimab (anti-PD-1) increased production of pro-inflammatory proteins important for anti-tumor immunity.4 In other preclinical studies, Fc-silent anti-TIGIT combined with anti-PD-1 also promoted tumor control without depleting other helpful immune cells, suggesting an Fc-enabled antibody may not be required for anti-tumor immunity.5
At the American Society of Clinical Oncology (ASCO) Annual Meeting in 2023, a Phase II clinical trial in non-small cell lung cancer (NSCLC) showed improvements in progression-free survival and overall response rates for those receiving domvanalimab plus zimberelimab versus zimberelimab alone. No unexpected safety signals were observed with the addition of domvanalimab to zimberelimab.6
In the November 2023 ASCO Virtual Plenary Series, data from a Phase II study in certain types of upper gastrointestinal (GI) cancer also showed encouraging early results in a population in which available immunotherapies have had limited long-term success.7
Changing the Treatment Paradigm
Together, our promising early data represent our commitment to exploring a novel way to target TIGIT in pursuit of changing the way combination treatments are used in immunotherapy. Everything we do is in service of one goal: improving care for people with cancer to help them live fuller lives. I take immense personal pride in contributing to Arcus’s mission.
Our researchers’ objective approach to evaluating the Fc activity hypothesis, combined with a significant amount of scientific intuition, led us to postulate that Fc activity does not appear to be needed for clinical benefit of anti-TIGIT antibodies. The development of domvanalimab is a testament to the extraordinary efforts of our talented team, who were recently recognized at the Pharmaceutical Technology Excellence Awards.
The entire Arcus team is passionately dedicated to continuing to investigate domvanalimab combinations in different cancer types with high unmet need.
Domvanalimab and zimberelimab are investigational molecules. Arcus has not received approval from any regulatory authority for any use globally, and their safety and efficacy for the treatment of lung or upper GI cancers have not been established.
References
1. Ge Zhouhong et al. “TIGIT, the next step towards successful combination immune checkpoint therapy in cancer.” Frontiers in Immunology 12 (2021): 699895.
2. He Xing, and Chenqi Xu. “Immune checkpoint signaling and cancer immunotherapy.” Cell research 30.8 (2020): 660-669.
3. Rotte Anand, Srikumar Sahasranaman, and Nageshwar Budha. “Targeting TIGIT for immunotherapy of cancer: Update on clinical development.” Biomedicines 9.9 (2021): 1277.
4. Anderson AE et al. “Preclinical characterization of AB154, a humanized a-TIGIT antibody, for use in combination therapies.” Poster Presentation, Society for Immunotherapy of Cancer, 2018.
5. Gauthier KE et al. “Anti-TIGIT Antibodies Promote Immune Activation Relevant to Targeting Stem-like and Tumor-specific T Cells in Combination With Anti-PD-1.” Poster Presentation, Immunology, 2022.
6. Johnson M et al. “ARC-7: Randomized Phase 2 Study of Domvanalimab + Zimberelimab +/- Etrumadenant vs Zimberelimab in First-Line, Metastatic, PD-L1-High Non-Small Cell Lung Cancer (NSCLC).” Rapid Plenary Presentation, American Society of Clinical Oncology, 2023.
7. Janjigian Y et al. “EDGE-Gastric Arm A1: Phase 2 study of domvanalimab, zimberelimab, and FOLFOX in first-line (1L) advanced gastroesophageal cancer.” American Society of Clinical Oncology Plenary Series Presentation, 2023.
RECENT ARTICLES
Combining for Change in Kidney Cancer
While there has been substantial progress in treatment in recent years, even more innovative approaches are needed to improve survival, especially when the cancer is metastatic, meaning it has spread to other parts of the body.
From Paper to Pill
There is opportunity to develop new and potentially improved molecules that block HIF-2α to further improve outcomes.

